Method for synthesizing 1,2-pentanediol
A synthesis method and a technology for pentanediol, applied in 1 field, can solve the problems of difficult product separation, serious environmental pollution, polybrominated substances, etc., and achieve the effects of low cost, good product quality and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
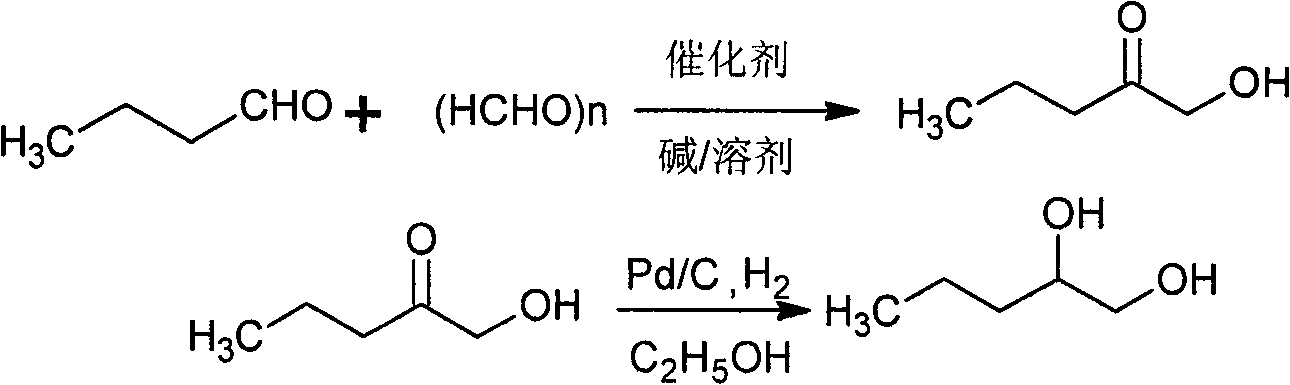
[0034] Embodiment 1: the first step reaction, in the 500ml reactor that thermometer is housed, stirrer, add n-butyraldehyde 36g (0.5mol), paraformaldehyde 22.5g (0.75mol), catalyst 3-ethylbenzothiazole 12.25 g (0.05 mol) of bromide salt, 5.05 g (0.05 mol) of triethylamine, 200 ml of absolute ethanol, under the protection of nitrogen gas, and react at a constant temperature of 70 ° C for 5 h. After the reaction, distill under reduced pressure to separate light fractions such as n-butyraldehyde and solvent, and rectify the residue under reduced pressure at 20 mmHg to obtain 33 g of intermediate product 1-hydroxyl-2-pentanone, with a yield of 65%, detected by GC Its purity is greater than 98%.
[0035] In the second step reaction, 33g (0.3166mol) of the intermediate product 1-hydroxyl-2-pentanone obtained in the previous step reaction, catalyst Pd / C0.33g, and absolute ethanol 150ml are put into a pressure reactor equipped with a thermometer and agitator In the process, vacuum fi...
Embodiment 2-5
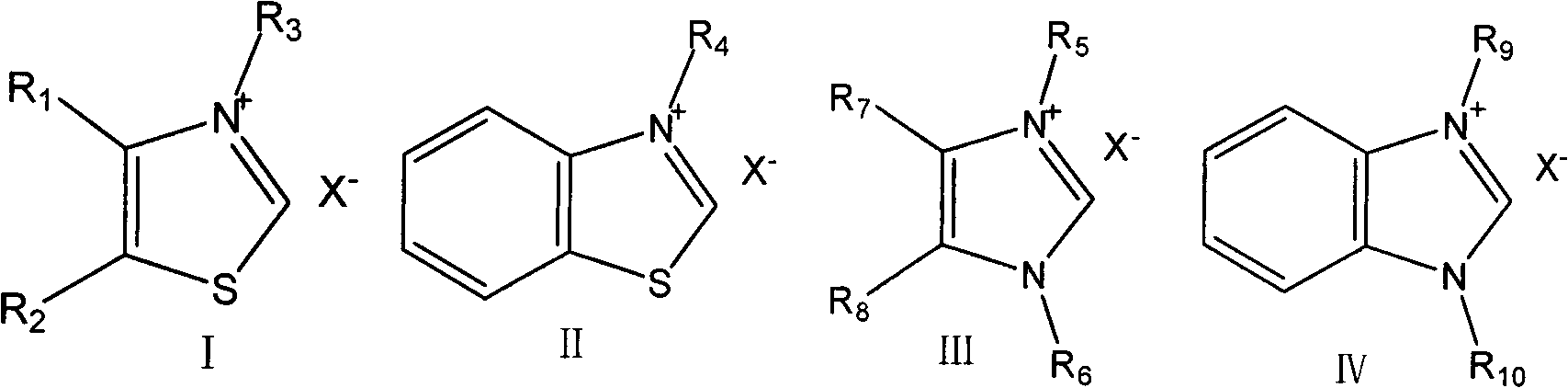
[0036] Embodiment 2-5: Same as Embodiment 1, the difference is that different thiazole (imidazole) salts are involved as catalysts for the first step reaction.
[0037] The first step reaction, feeding: n-butyraldehyde 36g (0.5mol), paraformaldehyde 22.5g (0.75mol), triethylamine 5.05g (0.05mol), absolute ethanol 200ml, 0.05mol catalyst A, B, C or d.
[0038] Operation steps Repeat the first step reaction. Nitrogen protection, constant temperature reaction at 70°C for 5h. After the reaction, low boiling point components such as the solvent were distilled off under reduced pressure, and the residue was distilled under reduced pressure at 20 mmHg to obtain the intermediate product 1-hydroxy-2-pentanone.
[0039] In the second step reaction, the intermediate product 1-hydroxy-2-pentanone is hydrogenated and reduced under the action of catalyst Pd / C, the reaction temperature is controlled at 40-50°C, the reaction time is 4h, and the product 1,2-pentanedi is obtained by vacuum di...
Embodiment 6-12
[0045] Embodiment 6-12: Same as Example 1, the difference is that sodium methoxide, sodium hydroxide, sodium carbonate, 1,8-diazacyclo[5,4,0]undecene-7 (DBU), Sodium acetate, pyridine, and N,N-dimethylethanolamine are used as bases in the first step reaction respectively.
[0046] The first step reaction, feeding: 36g (0.5mol) of n-butyraldehyde, 22.5g (0.75mol) of paraformaldehyde, 12.25g (0.05mol) of 3-ethylbenzothiazole bromide, 200ml absolute ethanol, 0.05mol methanol Sodium, Sodium Hydroxide, Sodium Carbonate, DBU, Sodium Acetate, Pyridine, or N,N-Dimethylethanolamine.
[0047]Operation steps Repeat the first step reaction. Nitrogen protection, constant temperature reaction at 70°C for 5h. After the reaction, low boiling point components such as the solvent were distilled off under reduced pressure, and the residual reactants were distilled under reduced pressure at 20 mmHg to obtain the intermediate product 1-hydroxyl-2-pentanone. GC detects its purity and calculates ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com