Recombinant human interleukin-12 preparation for injection and preparation method thereof
A technology for interleukin and injection, which is applied in the field of preparation and preparation, recombinant human interleukin-12 preparation and preparation for injection, and can solve hepatitis, hepatitis C, AIDS), various tumors (such as melanoma) , kidney cancer, rectal cancer, prostate cancer, cervical cancer, etc.), autoimmune diseases and asthma, etc., to achieve the effects of good appearance, low water content and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
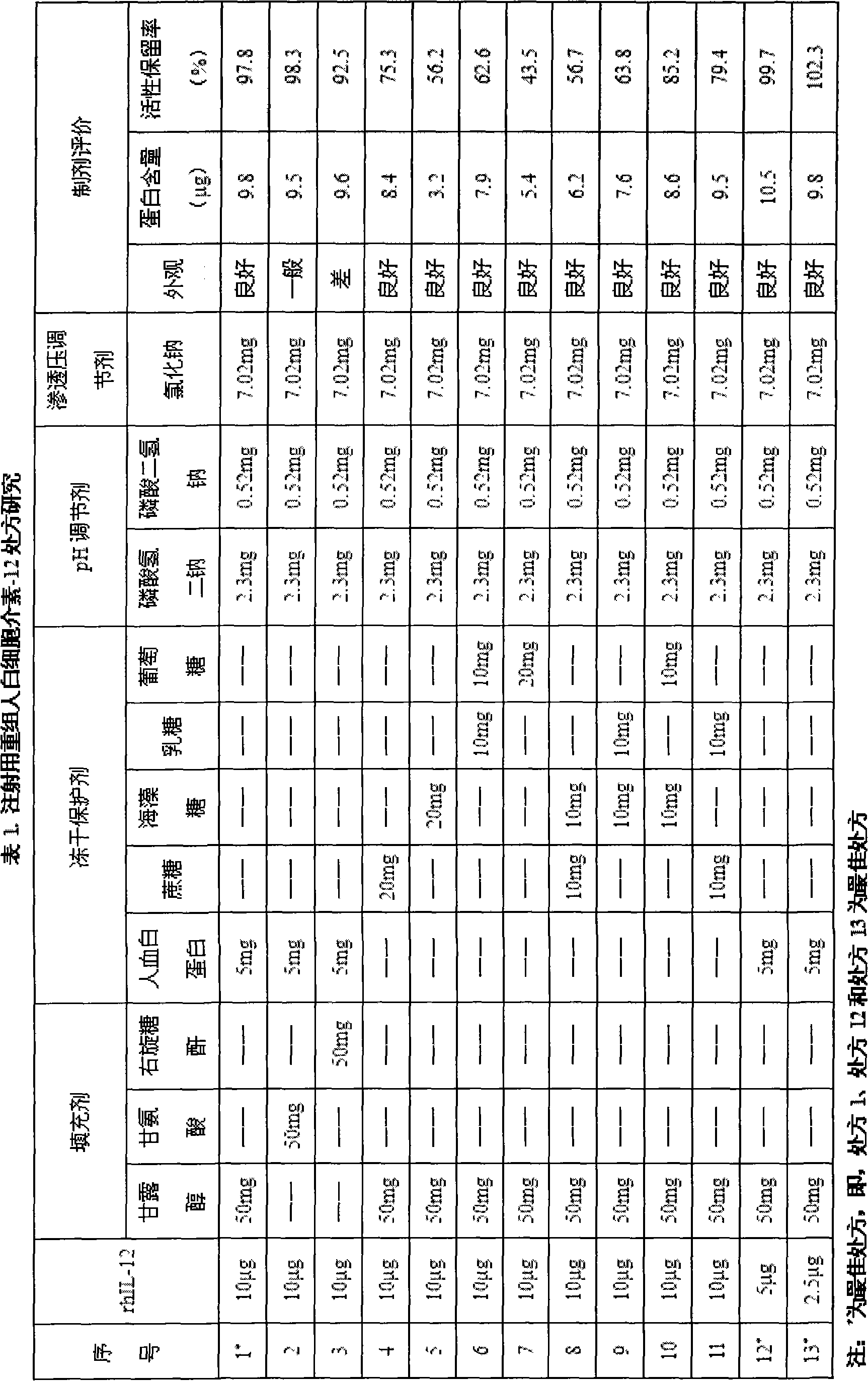
[0020] Clinical studies have shown that the clinical dose of recombinant human interleukin-12 (rhIL-12) is 1-500ng / kg, but at the dose level of 300-500ng / kg, some people show toxic side effects such as abnormal liver function and oral ulcers , the relatively safe and effective dose is 50ng-250ng / kg, and according to the weight of Chinese people around 50-60kg, the single bottle specification of this product is: rhIL-122.5, 5 or 10μg / preparation unit.
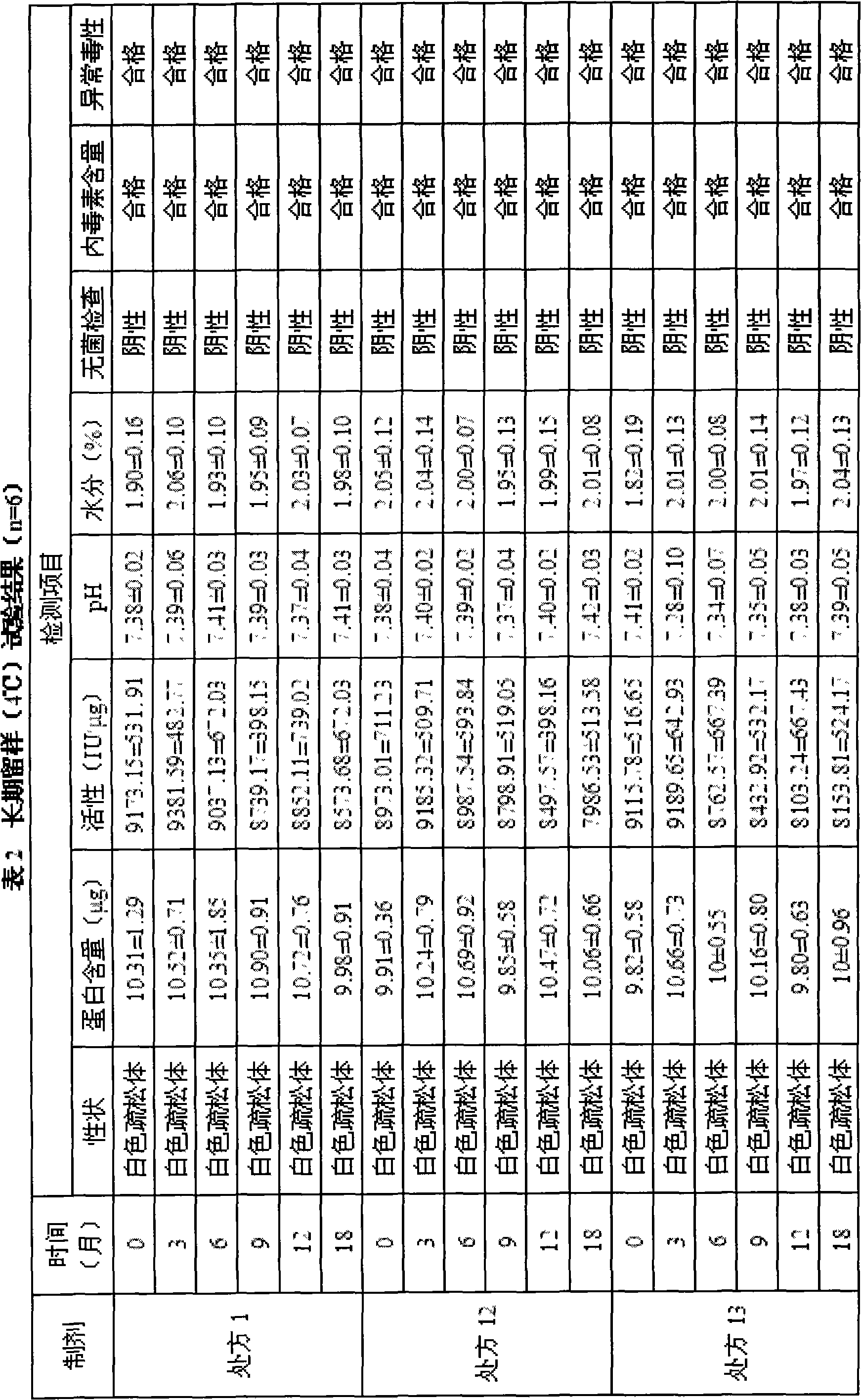
[0021] The preparation formed by the preparation formula is a freeze-dried powder injection, and the appearance and properties of the freeze-dried powder, as well as the determination of rhIL-12 content and biological activity are used as the screening basis when the formula is screened.
[0022] rhIL-12 is a glycoprotein formed by combining two subunits through disulfide bonds. It is easily soluble in water and stable in phosphate buffer at pH 7.4. Therefore, disodium hydrogen phosphate and sodium dihydrogen phosphate were used ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com