Perfluorosulfonic acidcontaining polyarylethersulfone proton exchange membrane material and preparation method thereof
The technology of perfluorosulfonic acid polyarylene ether and fluorosulfonic acid polyarylene ether is applied in the field of polyaryl ether sulfone polymer proton exchange membrane material and its preparation, and can solve the problems of high price, low α-relaxation temperature and the like, Achieve the effects of low mechanical properties, excellent mechanical properties, and high proton conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
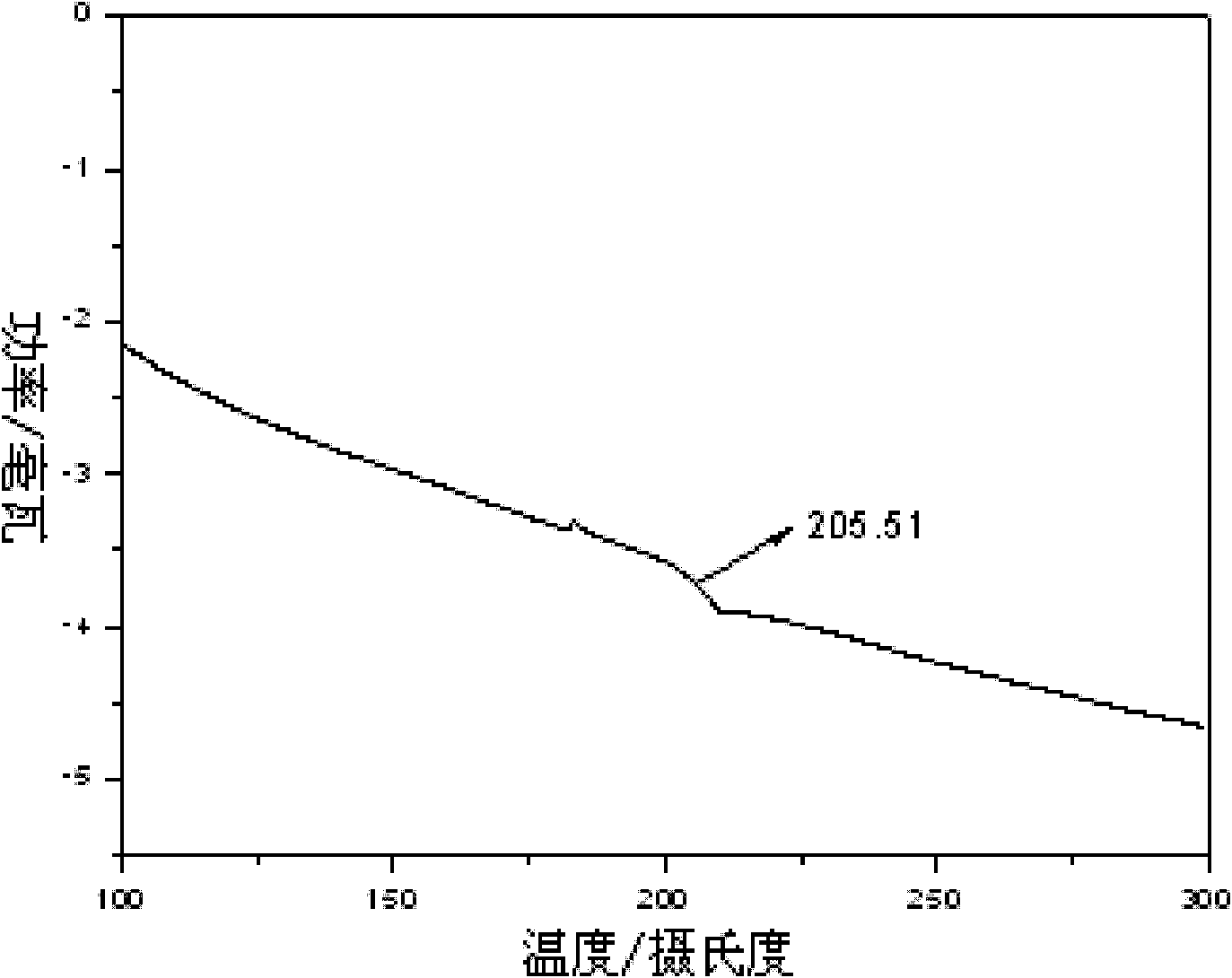
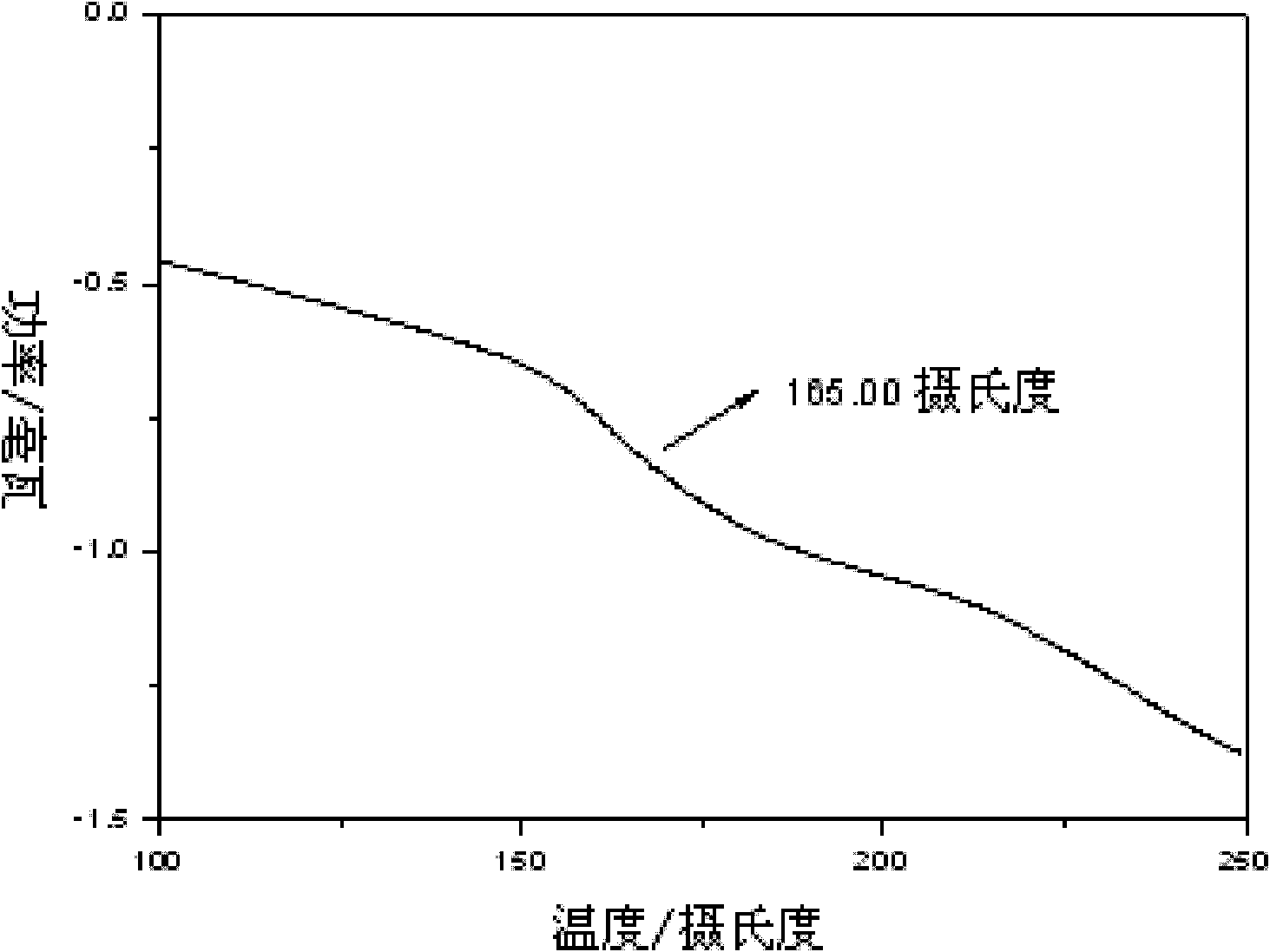
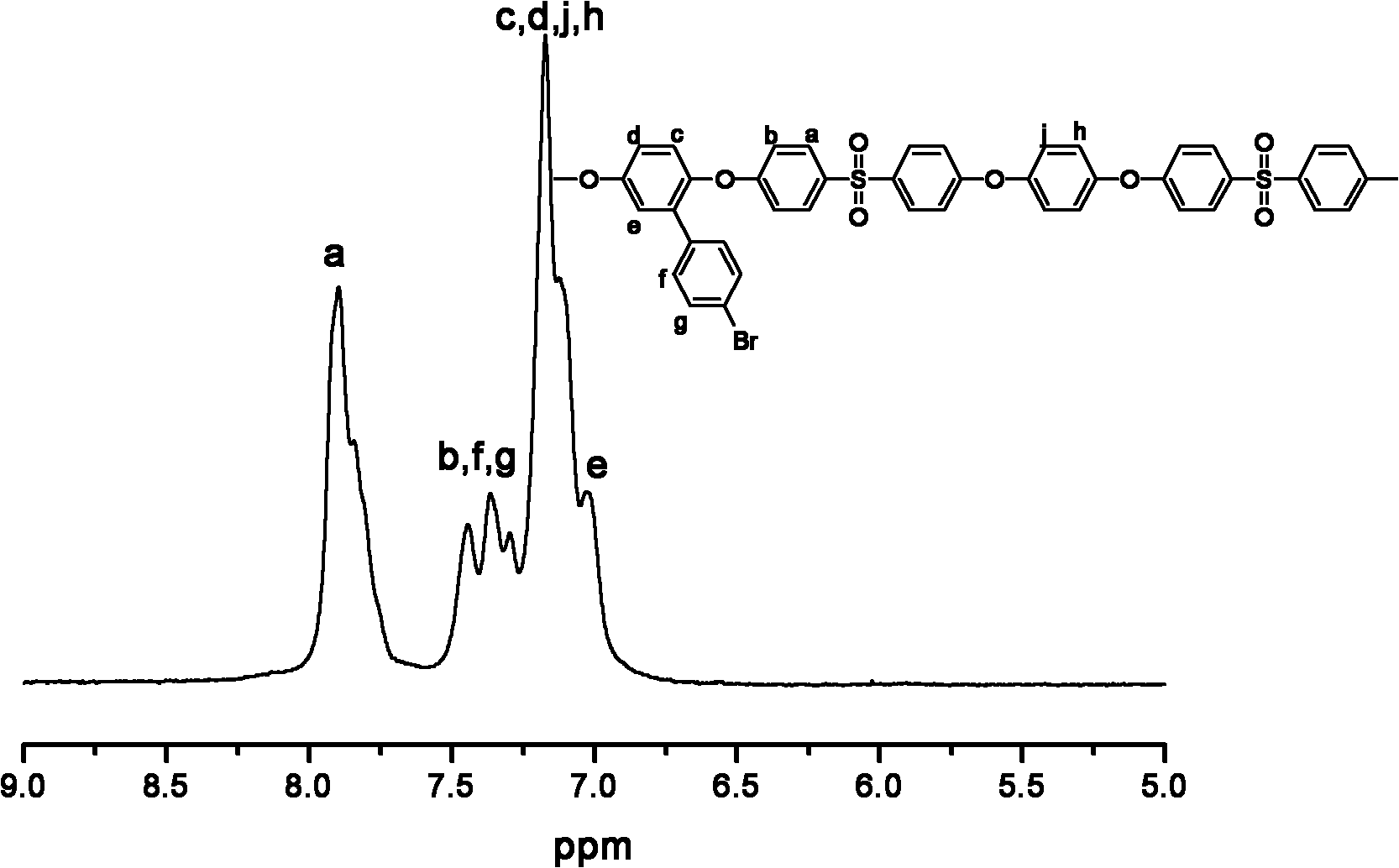
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of polyarylethersulfone containing 50% bromophenyl side groups (1)
[0027] 1.3255 grams of 2-(4'-bromophenyl)-1,4-hydroquinone monomer (m=0.005mol), 0.5506 grams of 1,4-hydroquinone monomer (n=0.005mol), 2.8716 grams of 4,4'-dichlorodiphenyl sulfone monomer (p=0.01mol) and 1.6585 grams of anhydrous potassium carbonate, 19 mL of sulfolane, and 10 mL of dehydrating agent (toluene or xylene) are put into a nitrogen through-hole, mechanical In the three-necked flask with stirring and water device, pass nitrogen gas, start stirring, raise the temperature to the reflux of the azeotropic dehydrating agent, react for 1 to 3 hours, remove the azeotropic dehydrating agent, raise the temperature to 190-210°C and continue the reaction for 7-10 hours; Then the obtained polymer solution was precipitated in deionized water, pulverized, washed, and dried to obtain 4 g of white polyarylethersulfone polymer powder with bromophenyl side groups.
[0028] The polyar...
Embodiment 2
[0034] Embodiment 2: the preparation (2) of polyaryl ether sulfone containing 100% bromophenyl side group
[0035] 2.6510 grams of 2-(4'-bromophenyl)-1,4-hydroquinone monomer (m=0.01mol), 2.8716 grams of 4,4'-dichlorodiphenyl sulfone monomer (p=0.01mol ) and 1.6585 grams of anhydrous potassium carbonate, 23mL sulfolane, and 12mL dehydrating agent (toluene or xylene) are put into a three-necked flask equipped with a nitrogen through hole, a mechanical stirrer and a water dispenser, nitrogen gas, stirring, and heating to a total temperature of Boil the dehydrating agent to reflux, react for 1-3 hours, remove the azeotropic dehydrating agent, raise the temperature to 190-210°C and continue the reaction for 7-10 hours; then precipitate the obtained polymer solution in deionized water, pulverize, wash and dry , to obtain 4 g of white polyaryl ether sulfone polymer powder with bromophenyl side groups.
[0036] The molecular formula is as follows:
[0037]
[0038] n=200-207.
...
Embodiment 3
[0043] Embodiment 3: the preparation (3) of polyaryl ether sulfone containing bromobenzene side group
[0044] 2-(4'-bromophenyl)-1,4-hydroquinone in Example 1 and 2 is replaced with 2-(2'-bromophenyl)-1,4-hydroquinone or 2 -(3'-Bromophenyl)-1,4-hydroquinone can also be used to obtain polyarylethersulfone polymers containing bromophenyl side groups.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com