Targeted anti-tumor recombinant protein and preparation method thereof
A recombinant protein and anti-tumor technology, applied in anti-tumor drugs, peptide/protein components, recombinant DNA technology, etc., can solve the problems of low protein renaturation rate, low expression, low activity, etc., to overcome low expression or inclusion The effects of body expression, improved affinity, and increased activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
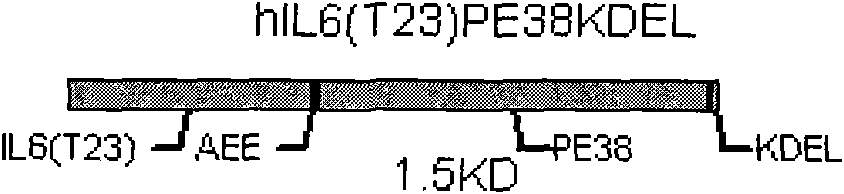
[0030] The following gene was artificially synthesized, its nucleotide sequence is SEQ No.3, and the recombinant targeting toxin hIL6(T23)-PE38KDEL of the following structure was expressed in E. coli cytoplasm by using the multiple cloning sites Nco I and Xhol I on pET28a, recombinantly expressed vector construction see Figure 4 . The recombinant plasmid was transferred into Rosstabule host bacteria, and induced with LB medium, 100uM IPTG, 28°C for 5 hours, and the expression amount accounted for about 14%-20% of the supernatant protein. After ultrafiltration with a molecular weight of 30,000MW and dialysis with 10mM PBS (pH7.4), the amino acid sequence is SEQ No.1.
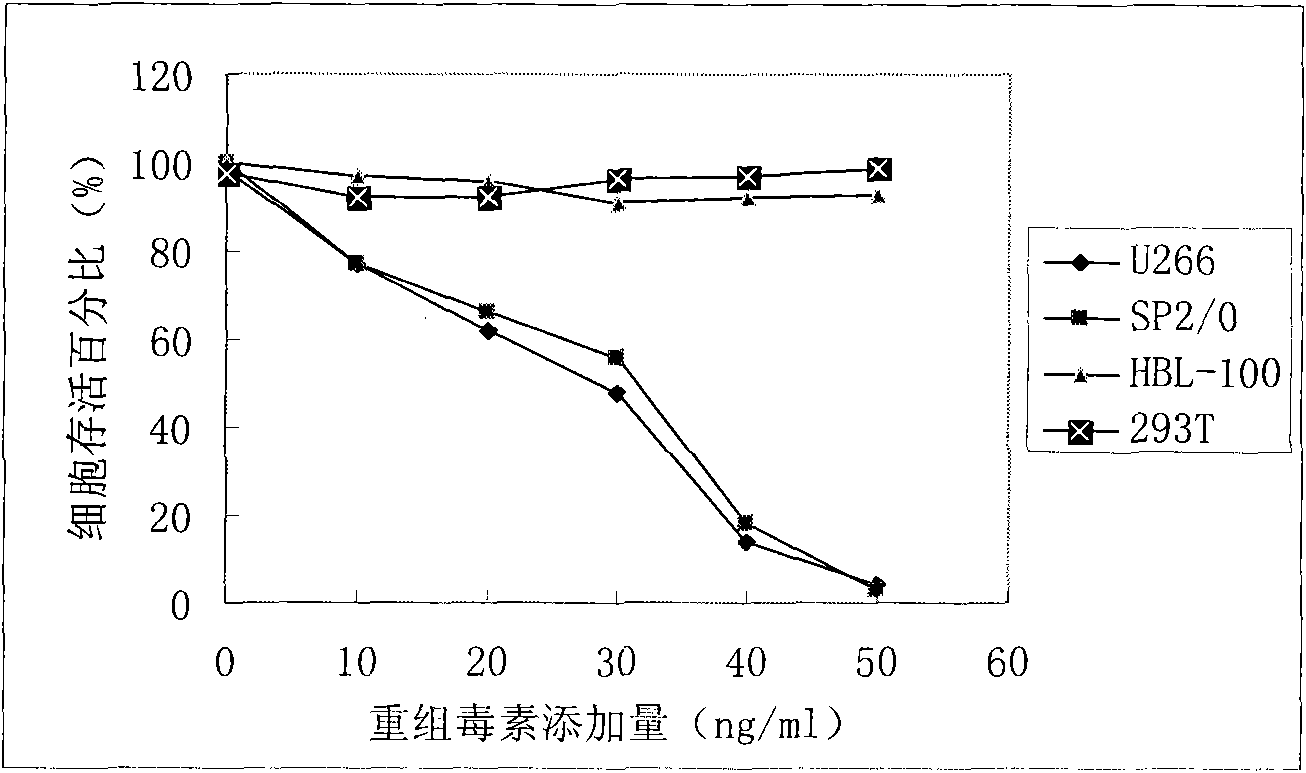
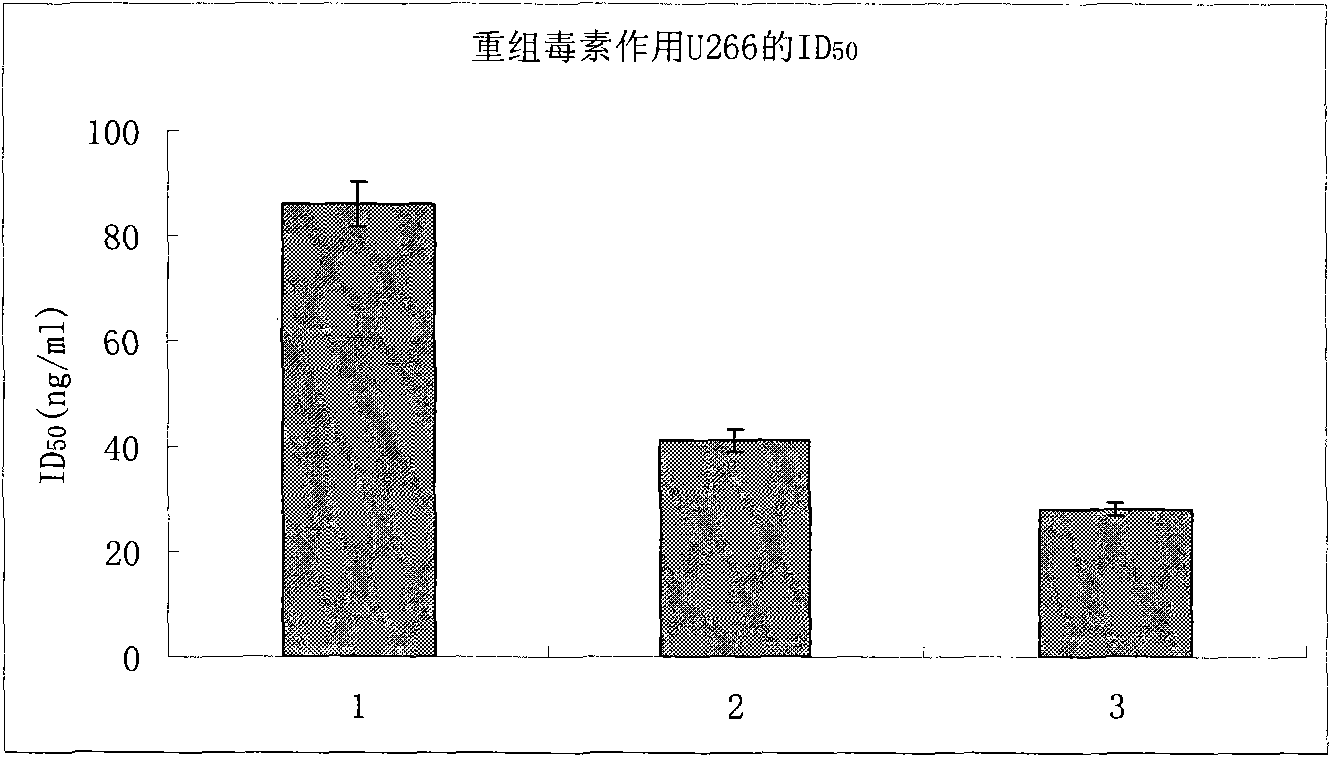
[0031] U266 cells and 293T cells were used to detect cell killing activity, and U266 had obvious killing activity, while 293T cells had no killing activity.
Embodiment 2
[0033] The following gene was artificially synthesized, its nucleotide sequence is SEQ No.3, using pET22b to secrete and express the recombinant targeting toxin hIL6(T23)-PE38KDEL with the following structure in Escherichia coli in the periplasmic space, see for recombinant expression vector construction Figure 5 . The recombinant plasmid was transferred into the Rosstabule host bacteria, induced with LB medium, 100uM IPTG, 28°C for 5 hours, and the periplasmic protein was extracted by the osmotic shock method, and the expression amount accounted for about 20%-25% of the supernatant protein. After ultrafiltration with a molecular weight of 30,000MW and dialysis with 10mM PBS (pH7.4), the amino acid sequence is SEQ No.1.
[0034] U266 cells and 293T cells were used to detect cell killing activity, and U266 had obvious killing activity, while 293T cells had no killing activity.
Embodiment 3
[0035] Embodiment 3: The following genes are artificially synthesized. The optimized recombinant toxin gene, whose nucleotide sequence is SEQ No.4, is inserted between the pGAPZa expression vector EcoRI and Kpn I. For the construction of the recombinant expression vector, see Figure 6 . The recombinant targeting toxin hIL6(T23)-PE38KDEL secreted and expressed in Pichia pastoris SMD1168 with the following structure was induced by YPD medium at 30°C for 72 hours, and the expression amount accounted for about 16% of the supernatant protein in the medium. After ultrafiltration with a molecular weight of 30,000MW and dialysis with 10mM PBS (pH7.4), the amino acid sequence is SEQ No.2.
[0036] U266 cells and 293T cells were used to detect cell killing activity, and U266 had obvious killing activity, while 293T cells had no killing activity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com