Application of IL-1ra-Fcepsilon fusion gene and coded protein thereof to treatment on allergic rhinitis
A technology of 1.il-1ra-fc and 2.il-1ra-fc is applied in the application field of treating allergic rhinitis, and can solve the problems of adverse effects, short half-life and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Preparation of IL-1ra-Fcε Fusion Gene Therapy Vector
[0022] The pIRES2-EGFP-IL-1ra-Fcε fusion gene expression vector [1] Transformed into Escherichia coli DH5α, cultivated in LB medium at 37°C vials, centrifuged at 9000rpm to collect bacteria, extracted a large amount of plasmids with the plasmid extraction kit from OMEGA company, concentrated the plasmids to more than 1mg / ml by ethanol precipitation, and stored at -20°C stand-by.
Embodiment 2
[0023] Example 2 Expression and protein purification of IL-1ra-Fcε fusion gene
[0024] The prokaryotic expression vector pBV220-IL-1ra-Fcε was transformed into Escherichia coli BL21, and the prokaryotic fermentation process was used to cultivate and induce Escherichia coli BL21 to express the recombinant protein. SPFF) to separate and purify the recombinant protein, and finally refold the protein by step-by-step dialysis [2] .
Embodiment 3
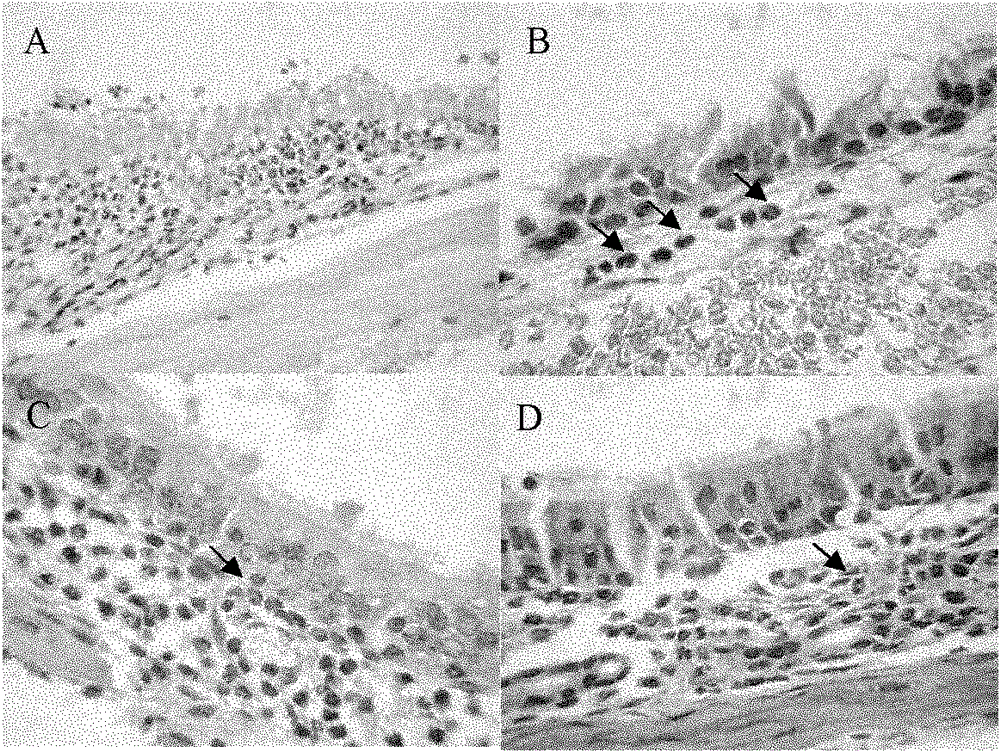
[0025] Embodiment 3 establishment of animal model [3,4]
[0026] Select healthy female Wistar rats weighing 135-165 g. On the first day, 1ml of normal saline, 1.5mg of egg protein, 3mg of aluminum hydroxide sol and 1×10 10 Inactivated pertussis bacilli were mixed and injected subcutaneously into the four paws of rats, and 0.2ml and 0.3ml were injected subcutaneously into each of the front and rear paws respectively. On the 5th day, 1ml of normal saline, 1.0mg of ovalbumin and 2mg of aluminum hydroxide sol were mixed and injected into the subcutaneous area of the back, divided into 5 points, and each point was injected with 0.2ml for a boost. The mice were challenged from the 14th day: 1% ovalbumin physiological saline solution was instilled daily, 30 μl in each nostril, once a day, 10 times in total.
[0027] Modeling was successful if the behavior (sneezes and nose scratches) and symptoms (nasal secretions, etc.) within 30 minutes after nasal drip challenge were greater ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com