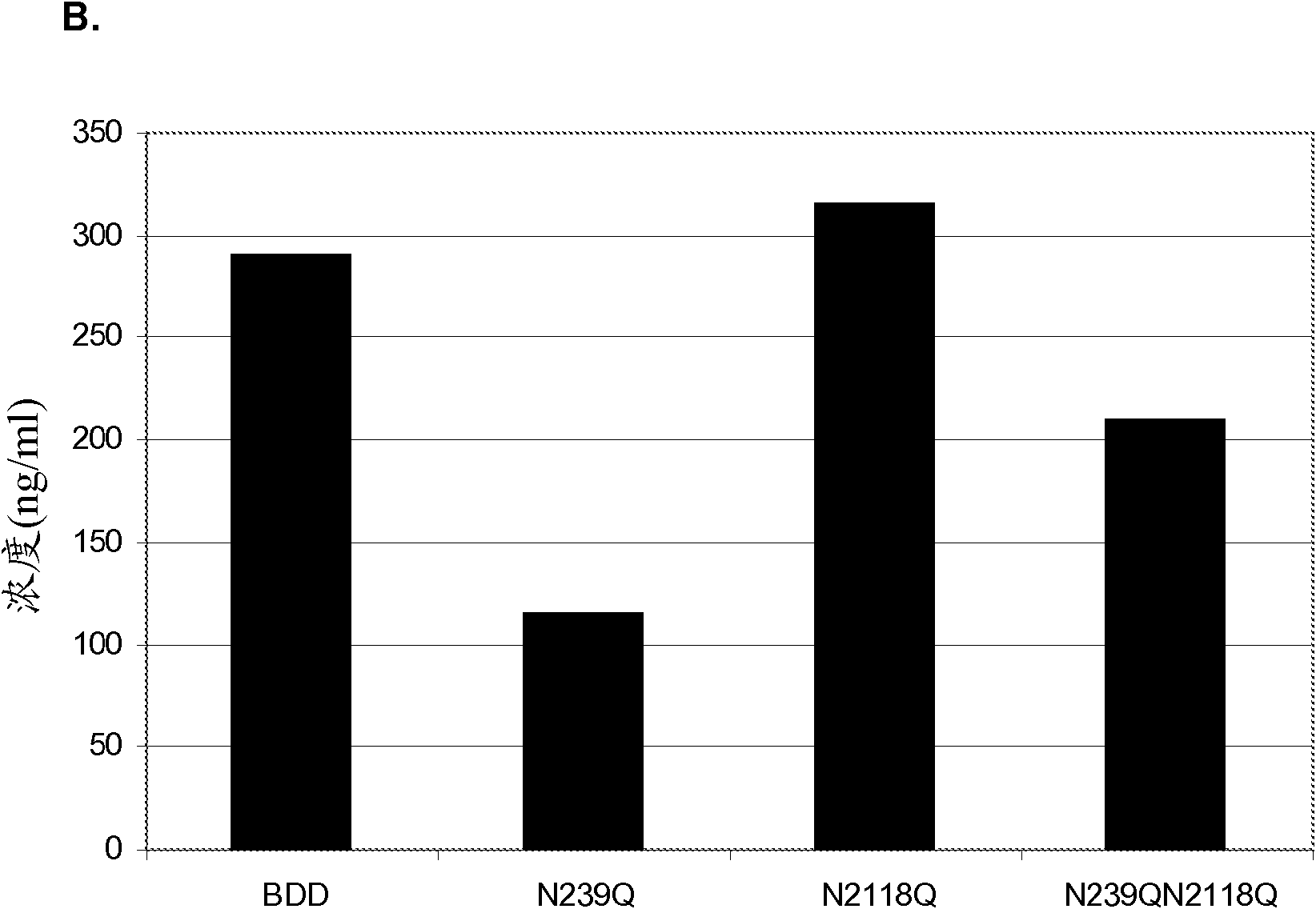Factor VIII muteins with reduced immunogenicity
A factor and recombinant factor technology, applied in the direction of factor VII, peptide/protein components, coagulation/fibrinolytic factor, etc., can solve the problems of reduced activity of recombinant protein and decreased productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
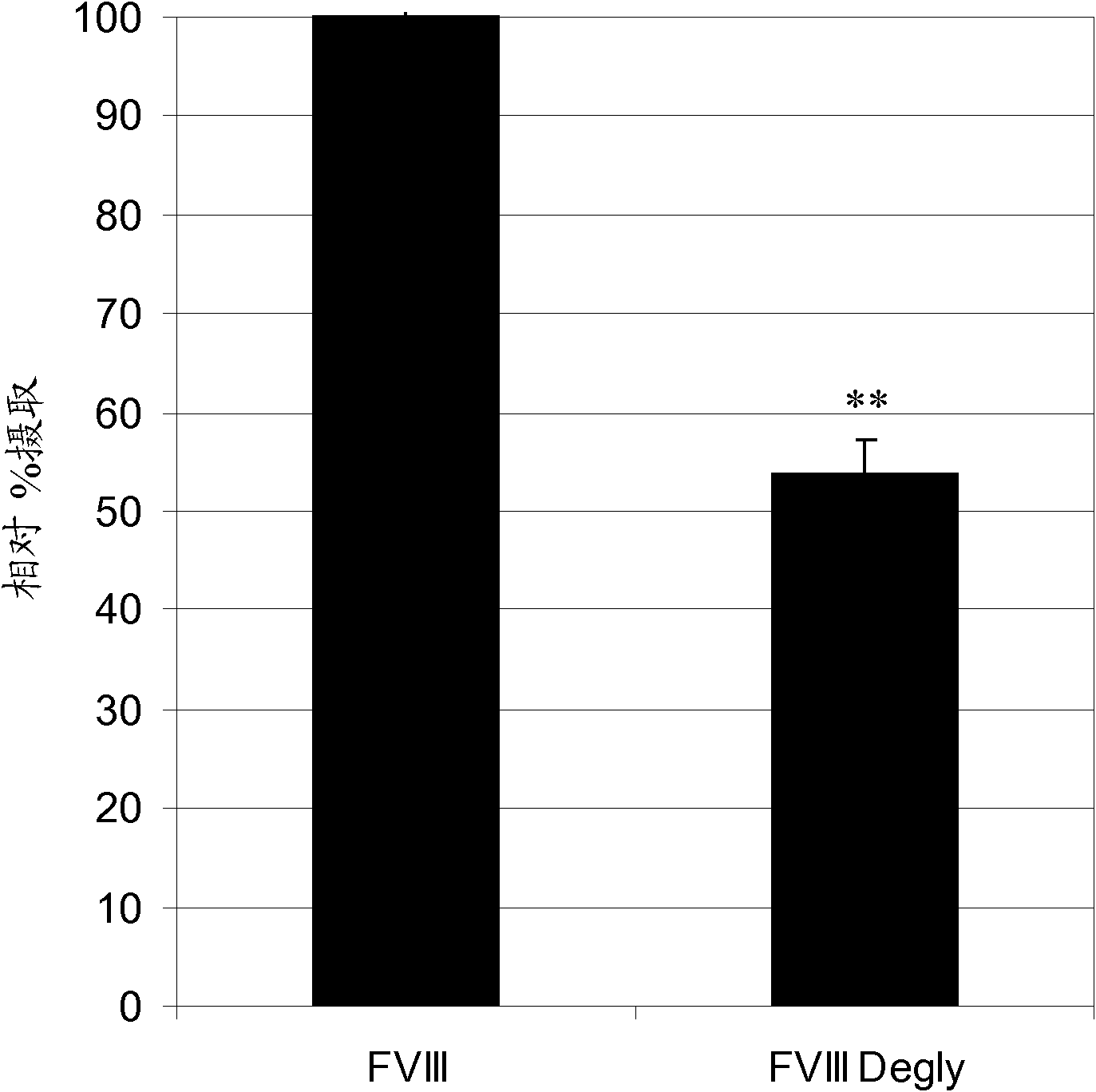
[0100] Example 1: Endocytosis of FVIII by dendritic cells
[0101] The effect of FVIII glycosylation on DC uptake in vitro was determined. Full-length rFVIII was first labeled for FACS analysis and then deglycosylated. To label rFVIII for FACS analysis, 6 μg of fluorescein isothiocyanate (FITC) in PBS (pH 9) was added to 100 μg of deglycosylated FVIII and allowed to mix for 2 hours at 4°C. By in 20mM HEPES, 150mM NaCl, 2% sucrose, and 100ppm Unconjugated FITC was removed by dialysis against -80 (polyethylene glycol sorbitan monooleate) solution (pH 7.5) at 4°C for 2 hours using a 50K membrane. FVIII concentration was quantified by Bradford assay and FVIII activity was determined by chromogenic assay. Tagged rFVIII is then enzymatically deglycosylated using endoglycosidase F1 (Endo-F1 ), which specifically cleaves N-linked oligosaccharides without denaturing the protein. rFVIII was incubated with Endo-F1 at 37°C for 1 hour. rFVIII was injected into 50K membranes and treat...
Embodiment 2
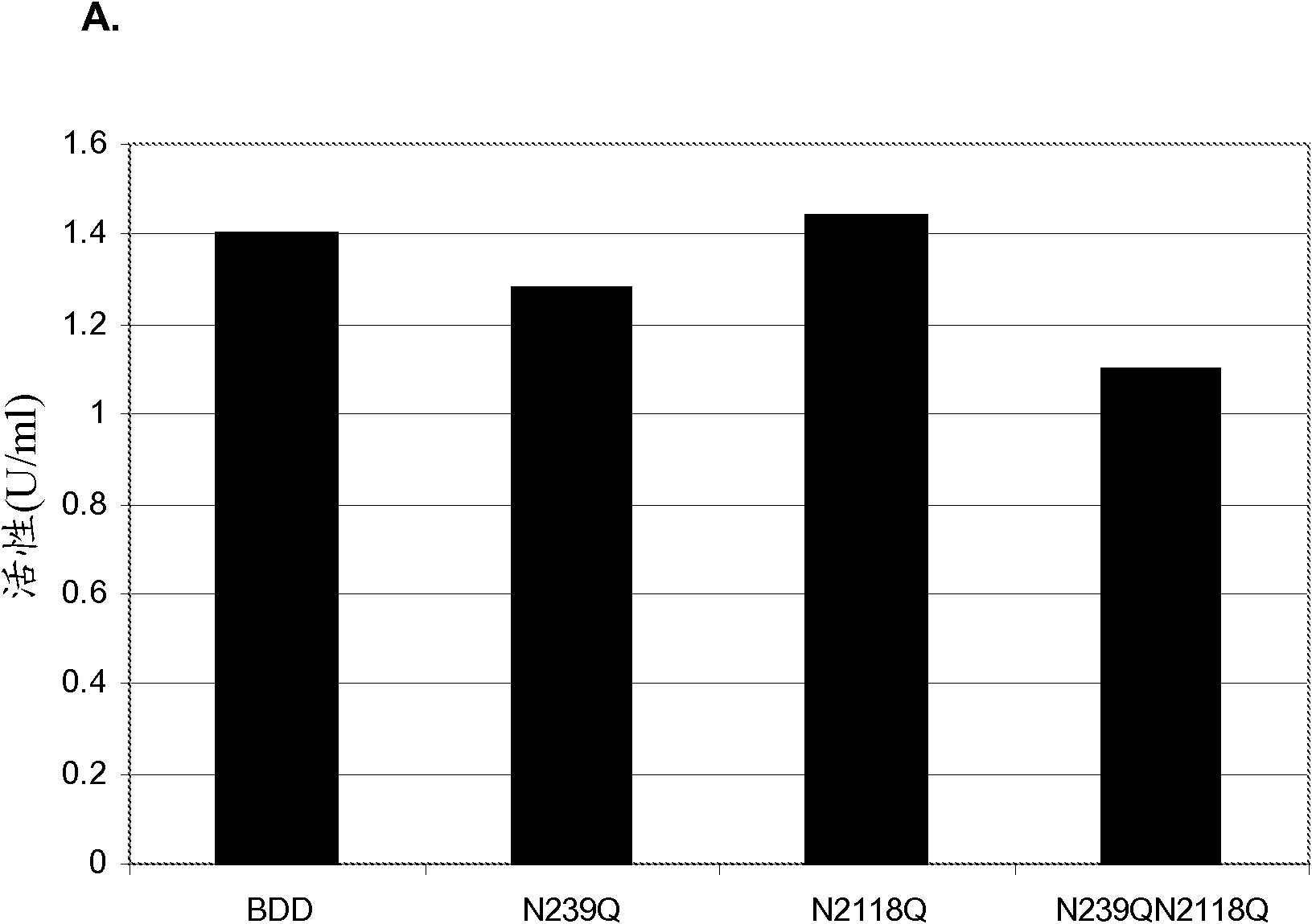
[0103] Example 2: Expression of FVIII mutein in HKB11 cells
[0104] BDD FVIII and three muteins of this BDD FVIII were expressed in HKB11 cells. BDD FVIII contains a deletion of almost 14 amino acids of the B domain such that the first 4 amino acids of the B domain are linked to the last 10 residues of the B domain. One BDD FVIII mutein contained a single glutamine-to-asparagine substitution at position 239 (N239Q), another contained a glutamine-to-asparagine substitution at position 2118 (N2118Q), and a third contained These two mutations (N239Q / N2118Q).
[0105] Using Lipofectamine TM 2000 (Invitrogen, Carlsbad, CA) HKB11 cells were transiently transfected with BDD FVIII and BDD FVIII mutein expression plasmids according to the manufacturer's instructions. HKB11 cells were transiently transfected with BDD and BDD mutein plasmids, and supernatants from these cells were tested for FVIII activity by chromogenic assay and FVIII concentration by ELISA. The specific activiti...
Embodiment 3
[0106] Example 3: Decreased uptake of FVIII muteins by dendritic cells
[0107] Because dendritic cell (DC) uptake of FVIII is thought to be mediated by the interaction of CD206 with the mannose-terminated glycans on FVIII, DC uptake of the N239Q / N2118Q BDD mutein was tested as described in Example 1 Ability. DCs were prepared as described above. DCs from two donors were pooled and then co-cultured with full-length rFVIII, BDD FVIII (described in Example 2), or the N239Q / N2118Q BDD mutein. Cells were co-cultured for 30 minutes in each well of a 96-well plate. The final volume per well was 100 μL, and the final concentration of rFVIII, BDD, or mutein was 10 nM. Plates were then incubated at 37°C for 30 minutes. A parallel uptake assay was also performed at 4°C as a control. Cells were pelleted by centrifuging the plate at 300 g for 5 minutes at 4°C. Aspirate the medium and replace with ice-cold PBS / 10mM EDTA / 0.01% -80 Wash the cells three times. Then pass through 25 μL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com