Recombinant promoters and vectors for protein expression in liver and use thereof
A promoter and recombinant nucleic acid technology, applied in the direction of peptide/protein components, animal/human proteins, using vectors to introduce foreign genetic materials, etc., can solve problems such as low-efficiency biosynthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154] Exemplary recombinant nucleic acid sequences encoding fVIII or fIX proteins, or variants thereof, modified for tissue-specific expression are discussed in Example 1.
[0155] SEQ ID NO: 12 provides an exemplary liver codon-optimized ET3 sequence. In some embodiments, there is provided a nucleic acid sequence comprising or having at least 90% (such as at least 95%) identity to or consisting of a nucleic acid sequence as shown in SEQ ID NO: 12 or A recombinant nucleic acid molecule consisting of a sequence having at least 90% (eg, at least 95%) identity therewith.
[0156] SEQ ID NO: 11 provides an exemplary CpG deleted liver codon optimized ET3 sequence. In some embodiments, there is provided a nucleic acid sequence comprising or having at least 90% (such as at least 95%) identity to or consisting of a nucleic acid sequence as shown in SEQ ID NO: 11 or A recombinant nucleic acid molecule consisting of a sequence having at least 90% (eg, at least 95%) identity therewith...
Embodiment approach
[0554] Clause 1: A vector comprising a liver-specific promoter nucleic acid sequence of less than 250 nucleotides operably combined with a heterologous nucleic acid sequence encoding a protein.
[0555] Clause 2: The vector according to Clause 1, wherein the liver-specific promoter sequence comprises a sequence greater than 50, 60, 70, 80, 85, 90, 95, 96, 97, Sequences with 98 or 99% sequence identity.
[0556] Clause 3: The vector according to Clause 1, wherein said liver-specific promoter sequence comprises a sequence greater than 50% to SEQ ID NO: 4, 5, 6, 7, 102, 103, 104, 105, 106, 107 or 108 , 60, 70, 80, 85, 90, 95, 96, 97, 98 or 99% sequence identity.
[0557] Clause 4: The vector according to Clause 1, wherein said protein is fVIII or fIX or a variant thereof.
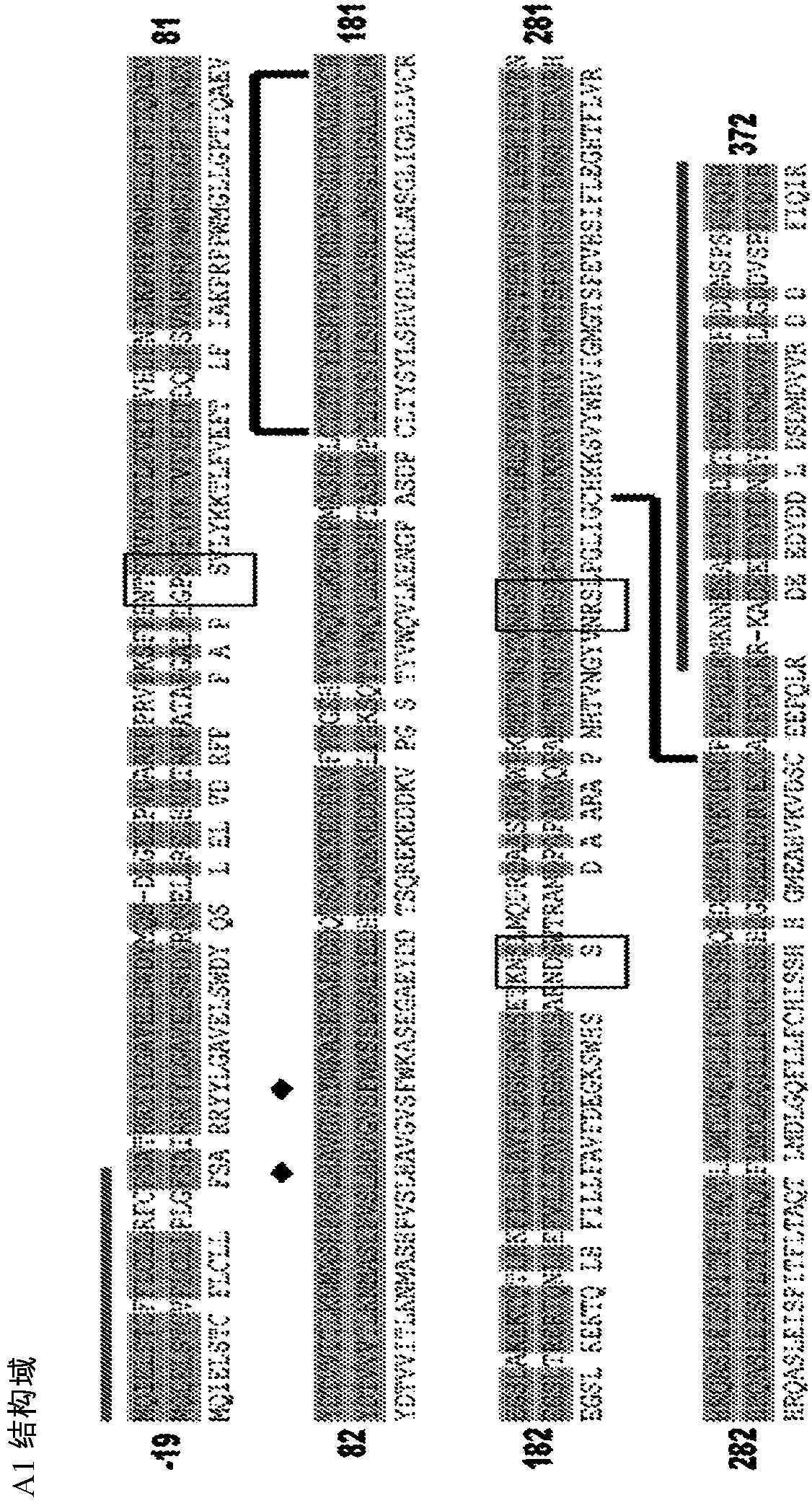
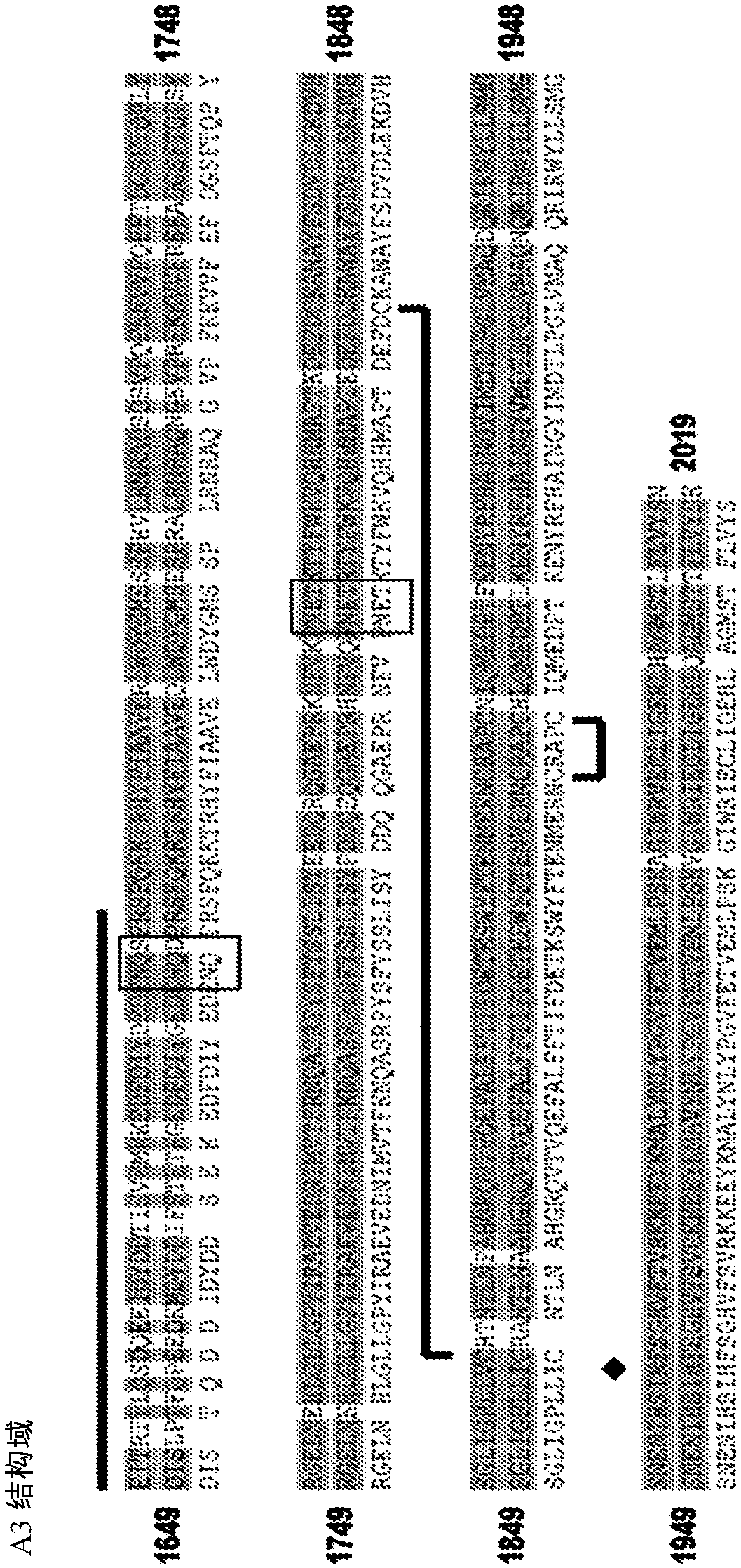
[0558] Clause 5: The vector according to clause 4, wherein the fVIII variant comprises an Al domain, an A2 domain, an ap domain, an A3 domain, a Cl domain and a C2 domain.
[0559] Clause 6: Vector accordin...
Embodiment
[0574] Example 1
[0575] Optimization of coagulation factors and their encoding DNA
[0576] This example illustrates optimization of the coding sequences of the fVIII and fIX proteins to increase their utility for in vivo expression and gene therapy.
[0577] The cDNA nucleotide sequences encoding fVIII and fIX were initially optimized by enforcing a codon usage bias specific for human hepatocytes compared to the nucleotides encoding the naturally occurring corresponding human non-codon-optimized sequences.
[0578] The adaptation of the nucleotide sequence encoding fVIII to the codon usage of human hepatocytes is expressed as the Liver Codon Adaptation Index (LCAI). The codon adaptation index was defined as a measure of the adaptability of the codon usage of genes relative to the codon usage of genes highly expressed in human liver. The relative fitness of each codon is the ratio of the usage of each codon to the usage of the most numerous codon among the same amino acids...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com