Thymalfasin sustained-release microsphere preparation and preparation method thereof
A new technology of sustained-release microsphere preparation and thymus method, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., and can solve problems such as difficulties in clinical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh 300mg of thymofasin and 200mg of gelatin and dissolve in 1ml of water for injection as the internal water phase; weigh 4g of glycolide-lactide copolymer and dissolve in 10ml of dichloromethane as the oil phase; add the oil phase to the internal In the water phase, use high-speed stirring (20000rpm) for 15 seconds to obtain colostrum and store it in an environment below 20 °C; add this colostrum to 5000 ml of 0.5% (W / V) polyvinyl alcohol under stirring The solution was double-emulsified, and the double-emulsion was continuously stirred for 3 hours to evaporate dichloromethane, centrifuged and washed to collect microspheres, the particle size of which was less than 100 microns, and the drug loading was 6.1%.
Embodiment 2
[0028] Weigh 500mg thymofasin and 300mg gelatin and dissolve in 2ml water for injection as the inner water phase; weigh 8g glycolide-lactide copolymer and dissolve it in 80ml dichloromethane as the oil phase; add the oil phase to the inner In the water phase, use an ultrasonic cell breaker (Branson S-250D) to perform ultrasonication for 10 seconds to obtain colostrum, which is stored in an environment below 20°C; add this colostrum to 8000 ml of 1.0% (W / V) polyvinyl alcohol solution to obtain double emulsion, the double emulsion was continuously stirred for 3 hours to evaporate dichloromethane, centrifuged and washed to collect microspheres, the particle size of the microspheres was less than 50 microns, and the drug loading was 4.5%.
Embodiment 3
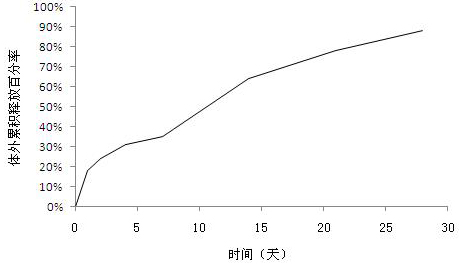
[0030] Determination of release in vitro of thymofaxin sustained-release microspheres
[0031] The Thymusfaxin slow-release microsphere preparation prepared by the above-mentioned examples was tested for release in vitro, and the assay method was as follows: accurately weigh 100 mg of the drug-containing microsphere and place it in a 10 ml stoppered test tube, and use pH 7.4 phosphate buffer ( Containing 0.02% sodium azide as a bacteriostatic agent, 0.05% Tween 80 as a wetting agent) 10ml as a release medium, placed in a constant temperature water bath shaker, at a shaking speed of 100rpm, and a temperature of 37°C±0.5°C. In vitro release assay of microspheres. On 1d, 2d, 4d, 7d, 14d, 21d, and 28d, 0.5ml of release medium was used to determine the content of thymus method by high performance liquid chromatography, and fresh release medium was added. figure 1 , 2 The in vitro cumulative release curves of the Thymofaxin sustained-release microsphere preparations prepared in E...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com