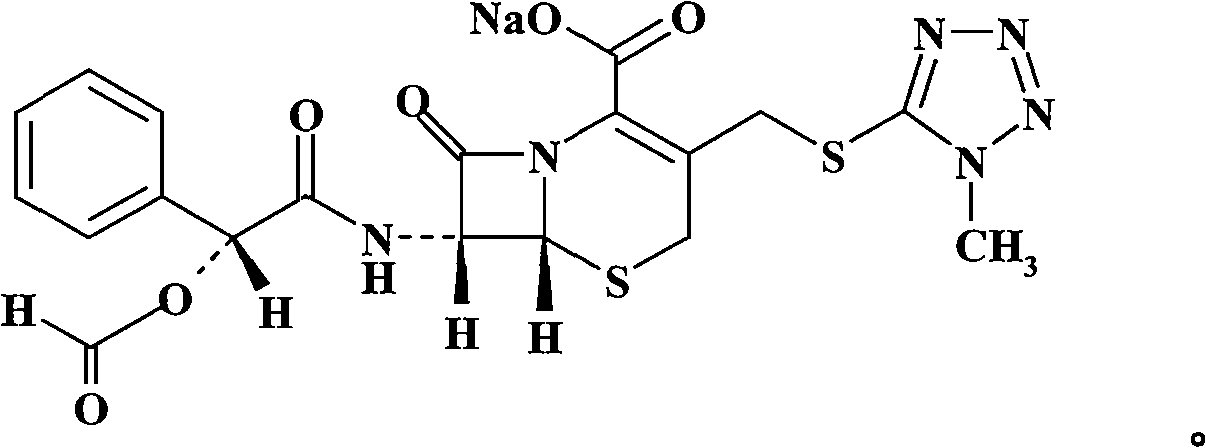
New method for preparing Cefamandole Nafate
A technology of cefamandole sodium and spamandole sodium, which is applied in the field of medicine and can solve the problems of low purity of cefamandole sodium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Accurately weigh 10 g of cefamandole sodium with a purity of 95% prepared according to US4351947. Add the solution containing cefamandole sodium into the fixed bed filled with D001 type strongly acidic styrene-based cation exchange resin, and continue the exchange until the pH value is adjusted to 3.3. Then, 10% hydrochloric acid aqueous solution was used as eluent for elution, and the eluate was collected and concentrated under reduced pressure. At this time, the purity detected by high performance liquid chromatography was 97.5%.
[0063] Then adjust the pH value to 6.8 with 1M aqueous sodium hydroxide solution when the temperature is raised to 50°C, and filter while hot to remove insoluble matter. At this time, the purity detected by high performance liquid chromatography was 98.4%.
[0064] Raise the temperature to 70°C and keep it for 20 minutes to evaporate the excess water in the cefamandole sodium solution, then add absolute ethanol in batches at a volume rati...
Embodiment 2
[0069] Accurately take by weighing the cefamandole sodium crude drug of 10g purity 97%. Add the solution containing cefamandole sodium into the fixed bed filled with D201 macroporous strongly basic styrene-based anion exchange resin, and continue the exchange until the pH value is adjusted to 2.8. Then 50% sulfuric acid solution was used as eluent for elution, and the eluate was collected and concentrated under reduced pressure. The purity detected by high performance liquid chromatography was 98.5%.
[0070] Then adjust the pH value to 7.5 with 2M aqueous sodium carbonate solution when the temperature is raised to 65°C, and filter while hot to remove insoluble matter. At this time, the purity detected by high performance liquid chromatography was 99.1%.
[0071] Raise the temperature to 80°C and keep it for 30 minutes to evaporate the excess water in the cefamandole sodium solution, then add absolute ethanol in batches at a volume ratio of water to ethanol of 4:6, and slowl...
Embodiment 3
[0076] Accurately take by weighing 10g the crude product cefamandole sodium of purity 96% that makes according to EP0432297. Add the solution containing cefamandole sodium into the reaction tank filled with GB / T 13659-2008 001×7 strongly acidic styrene-based cation exchange resin, and pass CO 2Gas facilitates the exchange until the pH is adjusted to 3.1. Then, 15% hydrochloric acid aqueous solution was used as eluent for elution, and the eluate was collected and concentrated under reduced pressure. At this time, the purity detected by high performance liquid chromatography was 97.9%.
[0077] Then adjust the pH value to 6.6 with 1M sodium acetate aqueous solution when the temperature is raised to 60°C, and filter while hot to remove insoluble matter. At this time, the purity detected by high performance liquid chromatography was 98.8%.
[0078] Raise the temperature to 80°C and keep it for 15 minutes to evaporate the excess water in the cefamandole sodium solution, then add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com