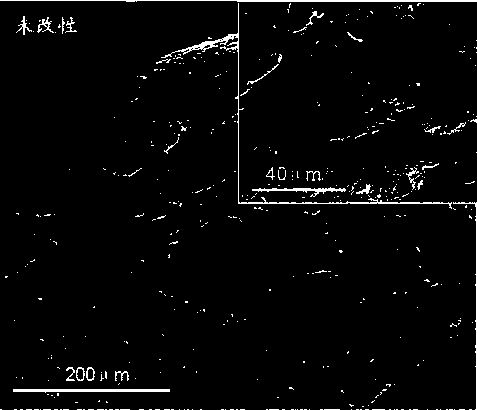
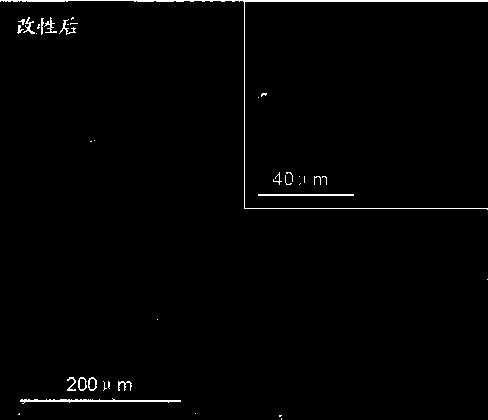
Method for fixing polypeptide aptamers on surface of cardiovascular implanting device
A technology of polypeptide aptamer and implantation device, which is applied in the field of surface modification of biological decontamination materials, can solve the problems of high late thrombosis risk, incomplete surface endothelialization, etc., and achieve the effect of excellent anticoagulant function.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A. Activation treatment
[0027] The sample was placed in 83% phosphoric acid solution, activated in an oven at 120°C for 0.5h, ultrasonically cleaned in deionized water, and dried. Then immerse the sample in 8.3% phosphoric acid solution for 10 minutes, keep it warm at 120°C for 12 hours, ultrasonically clean it with deionized water for 3 times, each time for 10 minutes, and dry it;
[0028] B. Silanization treatment:
[0029] The implanted device after the activation treatment in step A was placed in a 5% APTES / anhydrous tetrahydrofuran solution (V / V) and refluxed for 5 hours. The sample was taken out and continued to reflux in anhydrous tetrahydrofuran for 5 hours. Ultrasonic cleaning, vacuum drying;
[0030] C. Immobilized biotin
[0031] Add 0.1 mg of biotin to 20 mL of 0.001 mol / L 2-morpholineethanesulfonic acid (MES) aqueous solution, and add 1×10 -5 mol N-hydroxysuccinimide (NHS), 1×10 -5 mol 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) to activate t...
Embodiment 3
[0062] A. Activation treatment
[0063] Soak the cardiovascular implant device in a 4mol / L NaOH solution at 90°C for 24h, take it out, place it in deionized water at 120°C for 0.5h, and dry it;
[0064] B. Silanization treatment:
[0065] The implanted device after the activation treatment in step A was placed in a 5% APTES / anhydrous tetrahydrofuran solution (V / V) and refluxed for 5 hours. The sample was taken out and continued to reflux in anhydrous tetrahydrofuran for 5 hours. Ultrasonic cleaning, vacuum drying;
[0066] C. Immobilized biotin
[0067] Add 0.1 mg of biotin to 20 mL of 0.001 mol / L 2-morpholineethanesulfonic acid (MES) aqueous solution, and add 1×10 -5 mol N-hydroxysuccinimide (NHS), 1×10 -5 mol 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) to activate the carboxyl group on biotin. The silanized sample was immersed in the above solution, reacted at room temperature for 4 hours, ultrasonically cleaned in deionized water, and dried in vacuum.
[0068]...
Embodiment 4
[0075] A. Activation treatment
[0076] Soak the cardiovascular implant device in a 4mol / L NaOH solution at 90°C for 24h, take it out, place it in deionized water at 60°C for 24h, and dry it;
[0077] B. Silanization treatment:
[0078] The implanted device after the activation treatment in step A was placed in a 5% APTES / anhydrous tetrahydrofuran solution (V / V) and refluxed for 5 hours. The sample was taken out and continued to reflux in anhydrous tetrahydrofuran for 5 hours. Ultrasonic cleaning, vacuum drying;
[0079] C. Immobilized biotin
[0080] Add 0.1 mg of biotin to 20 mL of 0.001 mol / L 2-morpholineethanesulfonic acid (MES) aqueous solution, and add 1×10 -5 mol N-hydroxysuccinimide (NHS), 1×10 -5 mol 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) to activate the carboxyl group on biotin. The silanized sample was immersed in the above solution, reacted at room temperature for 4 hours, ultrasonically cleaned in deionized water, and dried in vacuum.
[0081] D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com