Partial peptide of lacritin
An amino acid and sequence technology, applied in peptide/protein components, animal/human proteins, peptide sources, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] The synthesis of embodiment 1 polypeptide
[0096] Polypeptides were synthesized by solid phase synthesis. Specifically, a fluorenylmethoxycarbonyl (Fmoc) group is introduced into an amino acid and the amino acid is supported with a resin. Then, using dichloromethane as solvent, 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethylurea (HBTU) and N-methylpyrrolidone (NMP) as crosslinking The coupling agent undergoes an amide bond forming reaction. The protecting group was removed using DMF / 20% piperidine. The resulting product was purified by high performance liquid chromatography (column: ODS, solvent: water / acetonitrile / 0.05% TFA). Thus, polypeptides 1-3 consisting of the following amino acid sequences were obtained.
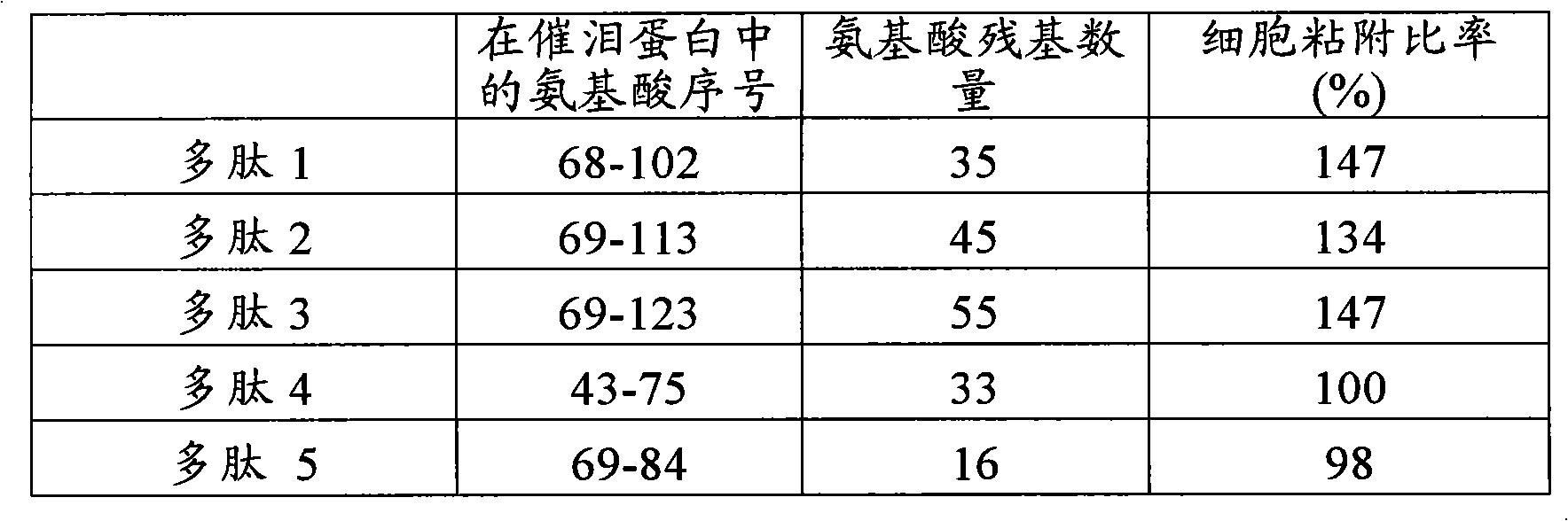
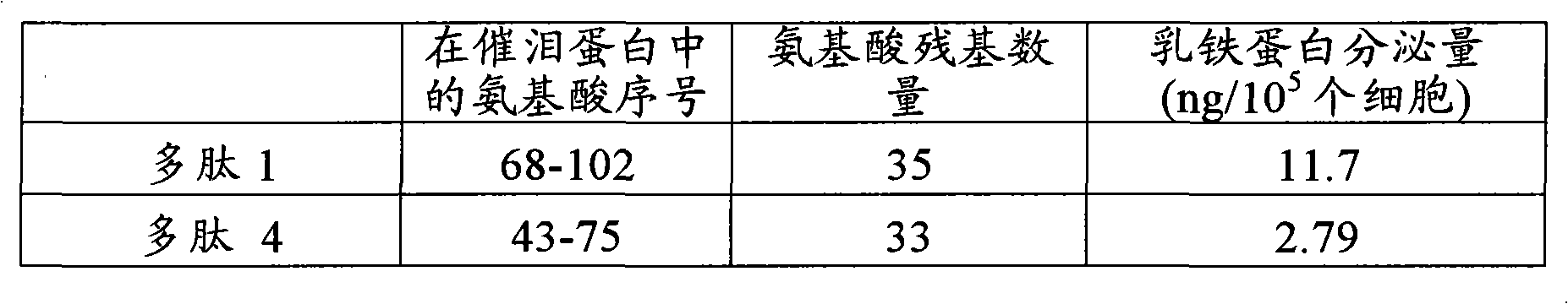
[0097] Polypeptide 1: VQGTAKVTSSRQELNPLKSIVEKSILLTEQALAKA (SEQ ID NO: 3)
[0098] White powder MALDI-TOF-MS calculated value: 3750.13; actual value: 3752.27; purity (HPLC A / A%) 96.207%
[0099] Peptide 2:
[0100] QGTAKVTS SRQELNPLKSIVEKSILLTEQALAKAGKGMHG...
Embodiment 2
[0105] The synthesis of embodiment 2 polypeptide
[0106] Polypeptides were synthesized by solid phase synthesis. Specifically, a fluorenylmethoxycarbonyl (Fmoc) group is introduced into an amino acid and the amino acid is supported with a resin. Also, using dichloromethane as solvent and 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethylurea (HBTU) and N-methylpyrrolidone (NMP) as crosslinking The coupling agent undergoes an amide bond forming reaction. The protecting group was removed using DMF / 20% piperidine. The resulting product was purified by high performance liquid chromatography (column: ODS, solvent: water / acetonitrile / 0.05% TFA). Thus, polypeptides 6 and 7 consisting of the following amino acid sequences were obtained.
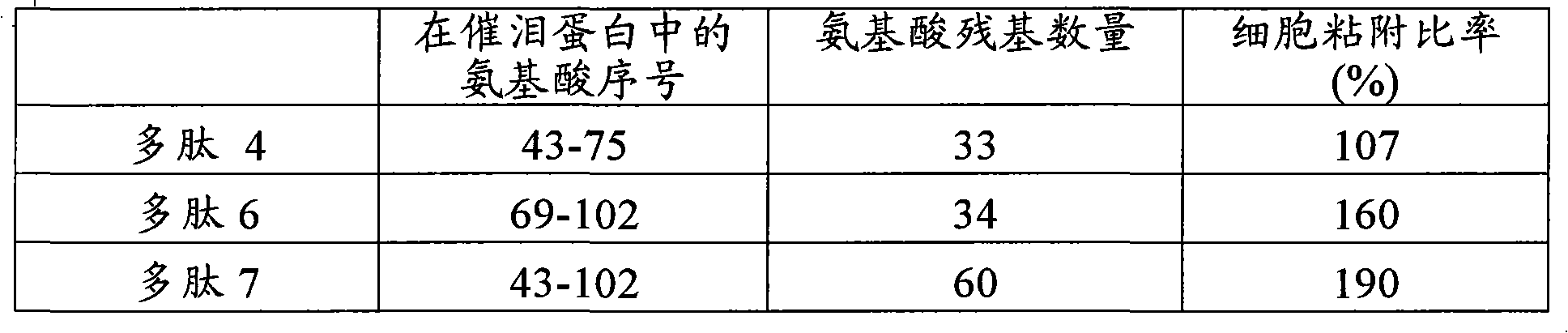
[0107] Peptide 6:
[0108] QGTAKVTSSRQELNPLKSIVEKSILLTEQALAKA (SEQ ID NO: 1)
[0109] White powder MALDI-TOF-MS calculated value: 3653.27; actual value: 3652.28; purity (HPLC A / A%) 99.26%
[0110] Peptide 7:
[0111] EISGPAEPASPETTTTAQETSAAAVQGTAK...
experiment Embodiment 1
[0119] Experimental Example 1: Facilitation of Partial Peptides of Lacrimal Protein on Adhesion of Human Corneal Epithelial Cells to Extracellular Matrix
[0120] An extracellular matrix solution (10 μg / mL, collagen type IV: Becton, Dickinson and Company) was added to a 96-well plate (Iwaki Glass Company, Limited). The solution was incubated at 37°C for 1 hour to coat the plate with extracellular matrix. After removing the excess extracellular matrix solution, 0.1% BSA solution (Sigma-Aldrich Co.) was added to block the area not coated with the extracellular matrix. Next, the BSA solution was removed and the plate was washed twice with PBS, and the polypeptides 1-5 (concentration: 100 μg / mL) synthesized in Example 1 and Comparative Example 1 were added at 50 μL per well. Moreover, the established human corneal epithelial cells (HCE-T: can be prepared by the method described in Invest Ophthalmol Vis Sci. 4 cells / 100 μL DMEM / F12 medium / well were added to the plate. Plates wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com