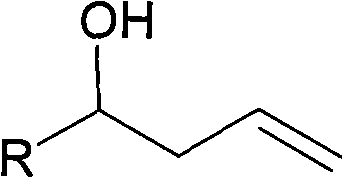
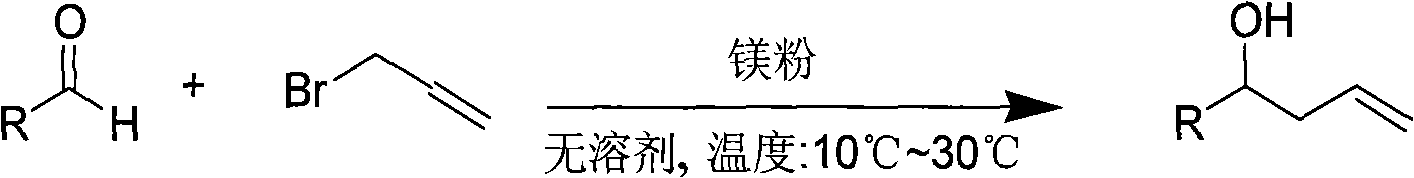
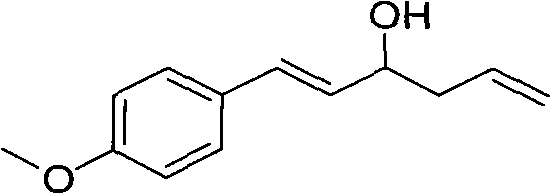
Method for preparing homoallylic alcohol
A high allyl alcohol, allyl alcohol technology, applied in the preparation of hydroxyl compounds, organic compounds, chemical instruments and methods, etc., can solve the problems of non-conformity with green chemistry, many side reactions, and inapplicability, and achieve Ease of industrial production, short reaction time, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1, the preparation of 1-(4-methoxyphenyl)-1-hexene-5-en-3-ol
[0022] (1) Activation of magnesium powder: put magnesium powder (2.4g, 0.1mol) and 80mL water into a 100mL round bottom flask equipped with a stirring bar, add 1mL concentrated hydrochloric acid dropwise within one minute and stir. After stirring for 10 min the water was decanted and the metal was washed sequentially with water (3 x 250 mL), acetone (3 x 150 mL) and diethyl ether (3 x 100 mL). Finally, the washed magnesium powder was transferred to a flask equipped with a vacuum conduit and dried in vacuum for three hours. It was used for experiments after cooling.
[0023] (2) Preparation of 1-(4-methoxyphenyl)-1-hexene-5-en-3-ol: add activated magnesium powder (0.096g ), add 4-methoxycinnamaldehyde (0.32g, 2mmol) via dropping funnel, allyl bromide (3mmol), stir at room temperature for 6 minutes; add saturated aqueous ammonium chloride solution (15ml ) after quenching the reaction, extracted w...
Embodiment 2
[0038] Embodiment two, the preparation of 1-(3-chlorophenyl)-3-buten-1-alcohol
[0039] (1) Activation of magnesium powder: with embodiment one.
[0040] (2) Preparation of 1-(3-chlorophenyl)-3-buten-1-ol: add activated magnesium powder (0.096g) and 3-chlorobenzene in a dry reaction flask (50mL round bottom flask) Formaldehyde (0.31g, 2mmol), allyl bromide (3mmol), stirred at room temperature for 5 minutes; after the reaction was completed, saturated ammonium chloride aqueous solution (15ml) was added to the reaction flask to quench the reaction, and extracted with diethyl ether (10ml), Stir for 10 minutes to terminate the reaction; separate the organic phase, extract the aqueous phase with diethyl ether (10mL) for 3 times, and then wash the combined organic phase with MgSO 4 Drying, evaporation of the solvent, and column chromatography (silica gel, 300-400; petroleum ether: ethyl acetate = 15:1), isolated pure product 1-(4-methoxyphenyl)-1-hexene- 5-en-3-ol. Yield: 87%.
[...
Embodiment 3
[0053] Embodiment three, the preparation of 1-(2,6-dichlorophenyl)-3-buten-1-ol
[0054] (1) Activation of magnesium powder: with embodiment one.
[0055] (2) Preparation of 1-(2,6-dichlorophenyl)-3-butene-1-alcohol:
[0056] Add activated magnesium powder (0.096g) and 2,6-dichlorobenzaldehyde (0.38g, 2mmol), allyl bromide (3mmol) into a dry reaction flask (50mL round bottom flask), and stir at room temperature for 5 minutes Add saturated ammonium chloride aqueous solution (15ml) to quench the reaction in the reaction flask after the end of the reaction, extract with diethyl ether (10ml), react under stirring for 10 minutes, and terminate the reaction; separate the organic phase, and use diethyl ether (10mL ) after extraction 3 times, the combined organic phases were washed with MgSO 4 Drying, evaporation of solvent, and column chromatography (silica gel, 300-400; petroleum ether: ethyl acetate = 15: 1), isolated pure product 1-(2,6-dichlorophenyl)-3-butene -1-ol. Yield: 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com