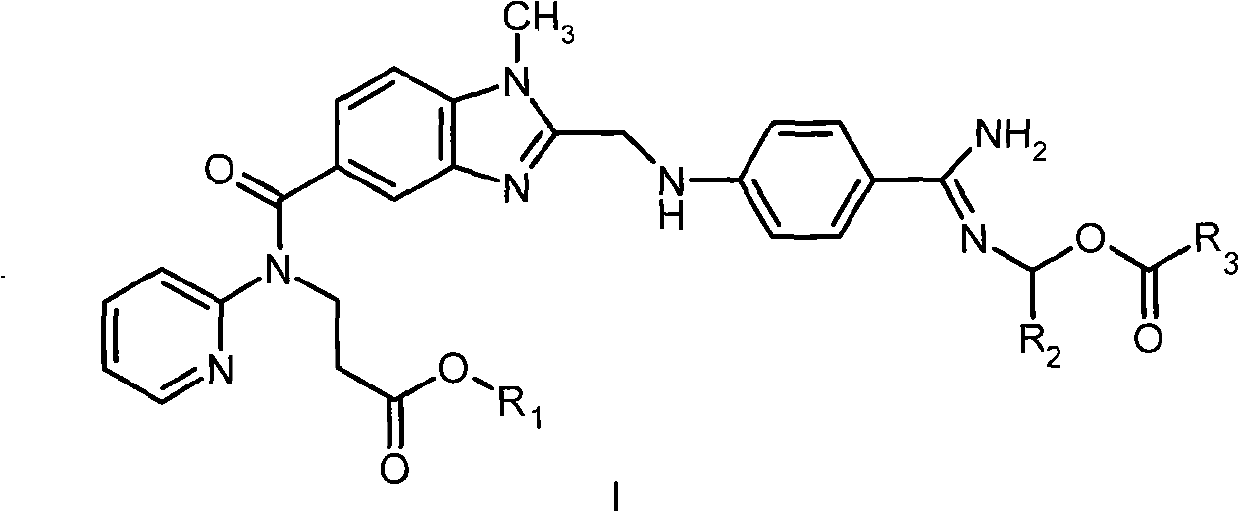
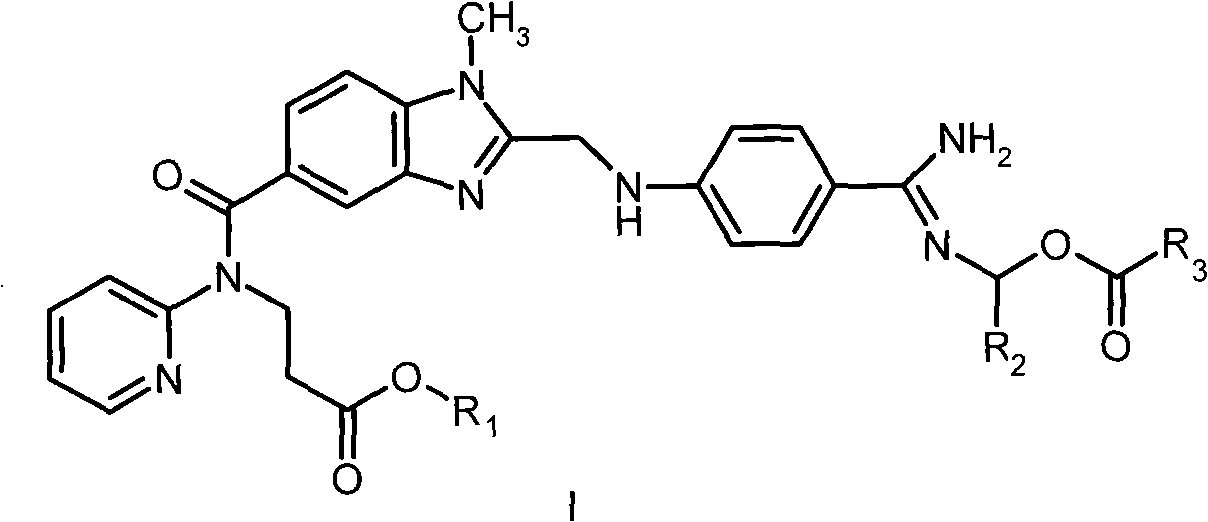
Dabigatran ester derivatives as prodrug
A technology of ester derivatives and pharmacy, which is applied in the field of ester derivatives of dabigatran as a prodrug, and can solve the problems that oral bioavailability needs to be further improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Example 1N-{[2-(((4-(N-Propionyloxymethyl-)amidino-phenyl)-amino)-methyl)-1-methyl-1H-benzimidazole-5- yl]-carbonyl}-N-(pyridin-2-yl)-β-alanine ethyl ester (I 18 ) preparation
[0122] 0.65 g (1.3 mmol) of dabigatran ethyl ester (II 1 ) was dissolved in 2 mL of DMF, and 0.18 g of K was added 2 CO 3 (1.3 mmol), 0.15 mL (1.3 mmol) of a solution of chloromethyl propionate in 1 mL of DMF was added dropwise with stirring, and the addition was completed within 15 min; after the addition, the reaction mixture was stirred at room temperature for 20 h. The reaction solution was concentrated in vacuo, and the residue was separated by silica gel column chromatography and eluted with a mixed solvent of dichloromethane: methanol (5: 1) to obtain 0.48 g of the target compound I 18 . 1 HNMRδ(ppm, DMSO-d 6 ): 1.08(t, 3H), 1.14(t, 3H), 2.34(q, 2H), 2.69(t, 2H), 3.78(s, 3H), 3.99(q, 2H), 4.24(t, 2H) , 4.68(d, 2H), 5.75(s, 2H), 6.90(d, 1H), 6.99(t, 1H), 7.15(m, 2H), 7.42(d, 1H), 7....
Embodiment 2
[0123] Example 2N-{[2-(((4-(N-butyryloxymethyl-)amidino-phenyl)-amino)-methyl)-1-methyl-1H-benzimidazole-5- yl]-carbonyl}-N-(pyridin-2-yl)-β-alanine ethyl ester (I 19 ) preparation
[0124] With reference to the method for embodiment 1, replace chloromethyl propionate and dabigatran ethyl ester (II) with chloromethyl-butyrate 1 ) reaction to obtain the target compound I 19 , the yield is 62%. 1 H NMRδ (ppm, DMSO-d 6 ): 0.85(t, 3H), 1.14(t, 3H), 1.51(m, 2H), 2.28(t, 2H), 2.69(t, 2H), 3.78(s, 3H), 3.99(q, 2H) , 4.24(t, 2H), 4.68(d, 2H), 5.75(s, 2H), 6.90(d, 1H), 6.99(t, 1H), 7.15(m, 2H), 7.42(d, 1H), 7.49 (d, 1H), 7.56 (dt, 1H), 7.82 (d, 2H), 8.42 (dd, 1H), 8.58-9.30 (bs, 2H).
Embodiment 3
[0125] Example 3N-{[2-(((4-(N-isobutyryloxymethyl-)amidino-phenyl)-amino)-methyl)-1-methyl-1H-benzimidazole-5 -yl]-carbonyl}-N-(pyridin-2-yl)-β-alanine ethyl ester (I 20 ) preparation
[0126] With reference to the method for embodiment 1, replace chloromethyl propionate and dabigatran ethyl ester (II) with chloromethyl-isobutyrate 1 ) reaction to obtain the target compound I 20 , the yield is 71%. 1 H NMRδ (ppm, DMSO-d 6 ): 1.05(d, 6H), 1.14(t, 3H), 2.55(m, 1H), 2.69(t, 2H), 3.78(s, 3H), 3.99(q, 2H), 4.24(t, 2H) , 4.68(d, 2H), 5.75(s, 2H), 6.89(m, 3H), 7.12(m, 2H), 7.36-7.60(m, 4H), 7.68(d, 2H), 8.41(m, 1H) ), 8.68 (s, 2H), 8.58-9.30 (bs, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com