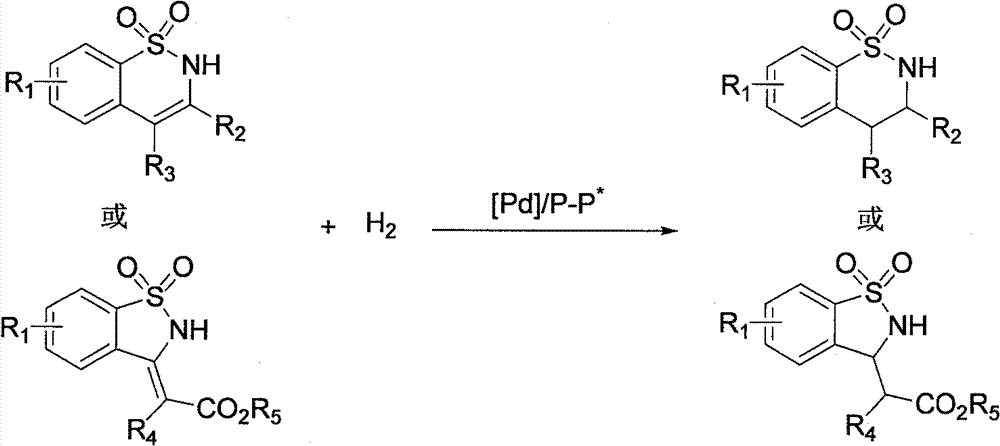
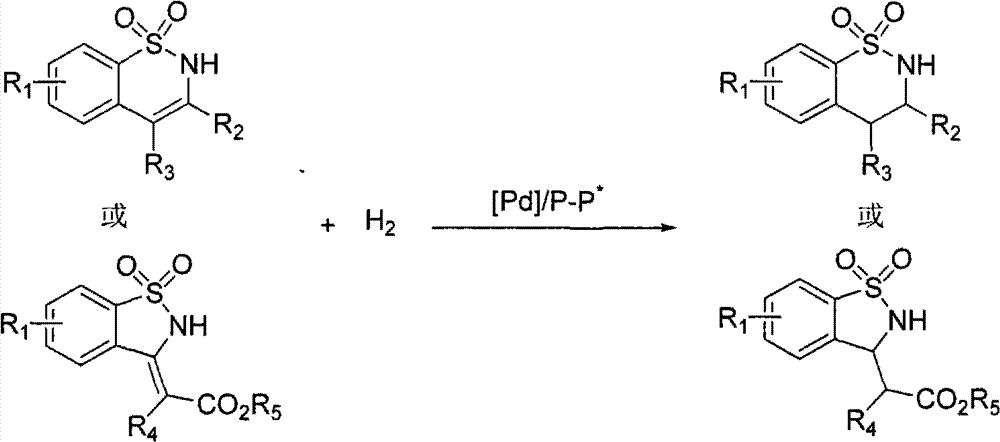Method for synthesizing chiral benzosultam via palladium-catalytic asymmetric hydrogenation
A benzosultam, asymmetric technology, applied in the field of cyclic sulfonamide compounds, achieves the effects of complete reaction, convenient preparation, high reactivity and enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: optimization of conditions
[0030] Put palladium trifluoroacetate (0.0025 mmol, 0.85 mg) and chiral ligand (0.003 mmol) into the reaction bottle, add 1 ml of acetone after nitrogen replacement, and stir at room temperature for 1 hour. Then concentrate in vacuo, add 2.5 milliliters of 2,2,2-trifluoroethanol under nitrogen, transfer this solution to the reaction kettle with substrate 1a (34 mg, 0.125 mmol) in advance, and feed hydrogen gas for 28 or 40 Atmospheric pressure, 50 or 70°C for 15 hours. Slowly release hydrogen, remove the solvent, and directly separate the pure product by column chromatography. The reaction formula and ligand structure are as follows:
[0031]
[0032] The conversion rate was determined by proton nuclear magnetic resonance spectroscopy, and the enantiomeric excess of the product was determined by chiral liquid chromatography, as shown in Table 1.
[0033] Table 1. Asymmetric hydrogenation of benzo six-membered cyclic sulfen...
Embodiment 2
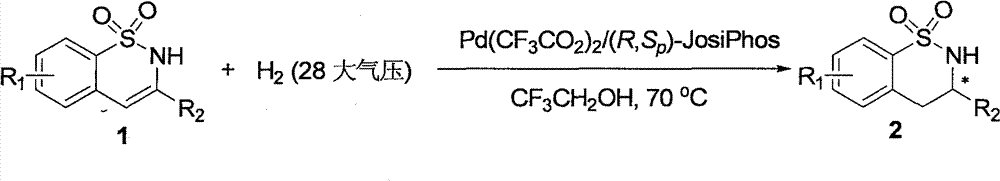
[0042] Example 2: Synthesis of various chiral benzosultams by palladium-catalyzed asymmetric hydrogenation 2
[0043] Drop into palladium trifluoroacetate (0.85 mg, 0.0025 mmol) and (R, S p )-JosiPhos (1.9 mg, 0.003 mmol) or (R, R p)-WalPhos (2.8 mg, 0.003 mmol), after nitrogen replacement, 1 ml of acetone was added, and stirred at room temperature for 1 hour. Then concentrate in vacuo, add 2.5 milliliters of 2,2,2-trifluoroethanol under nitrogen, transfer this solution to a reaction kettle with substrate (0.125 mmol) in advance, feed hydrogen to 28 atmospheres, and react at room temperature for 15 - 24 hours, slowly releasing hydrogen. After removing solvent, direct column chromatography separates and obtains pure product, and reaction formula is as follows:
[0044]
[0045] The enantiomeric excess of the product was determined by chiral liquid chromatography, see Table 2.
[0046] Table 2. Palladium-catalyzed asymmetric hydrogenation for the synthesis of various chir...
Embodiment 3
[0049] Example 3: Synthesis of various chiral benzopenta-sultams 4 by palladium-catalyzed asymmetric hydrogenation
[0050] Palladium trifluoroacetate (0.85 mg, 0.0025 mol) and (S,S)-f-Binaphane (2.4 mg, 0.003 mmol) were put into the reaction flask, 1 ml of acetone was added after nitrogen replacement, and stirred at room temperature for 1 hour. Then concentrate in vacuo, add 2.5 milliliters of 2,2,2-trifluoroethanol under nitrogen, transfer this solution to a reaction kettle with substrate (0.125 mmol) in advance, feed hydrogen to 40 atmospheres, and react at room temperature for 15 Hours, slowly release hydrogen. After removing solvent, direct column chromatography separates and obtains pure product, and reaction formula is as follows:
[0051]
[0052] R 1 is H or methyl, R 3 is methyl, ethyl or tert-butyl.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com