Pharmaceutical preparation
A technology for pharmaceutical preparations and renin inhibitors, applied in the field of pharmaceutical preparations, can solve the problems of not considering drug characteristics and absorption principles, and achieve the effect of improving the problem of simultaneous administration and effective curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
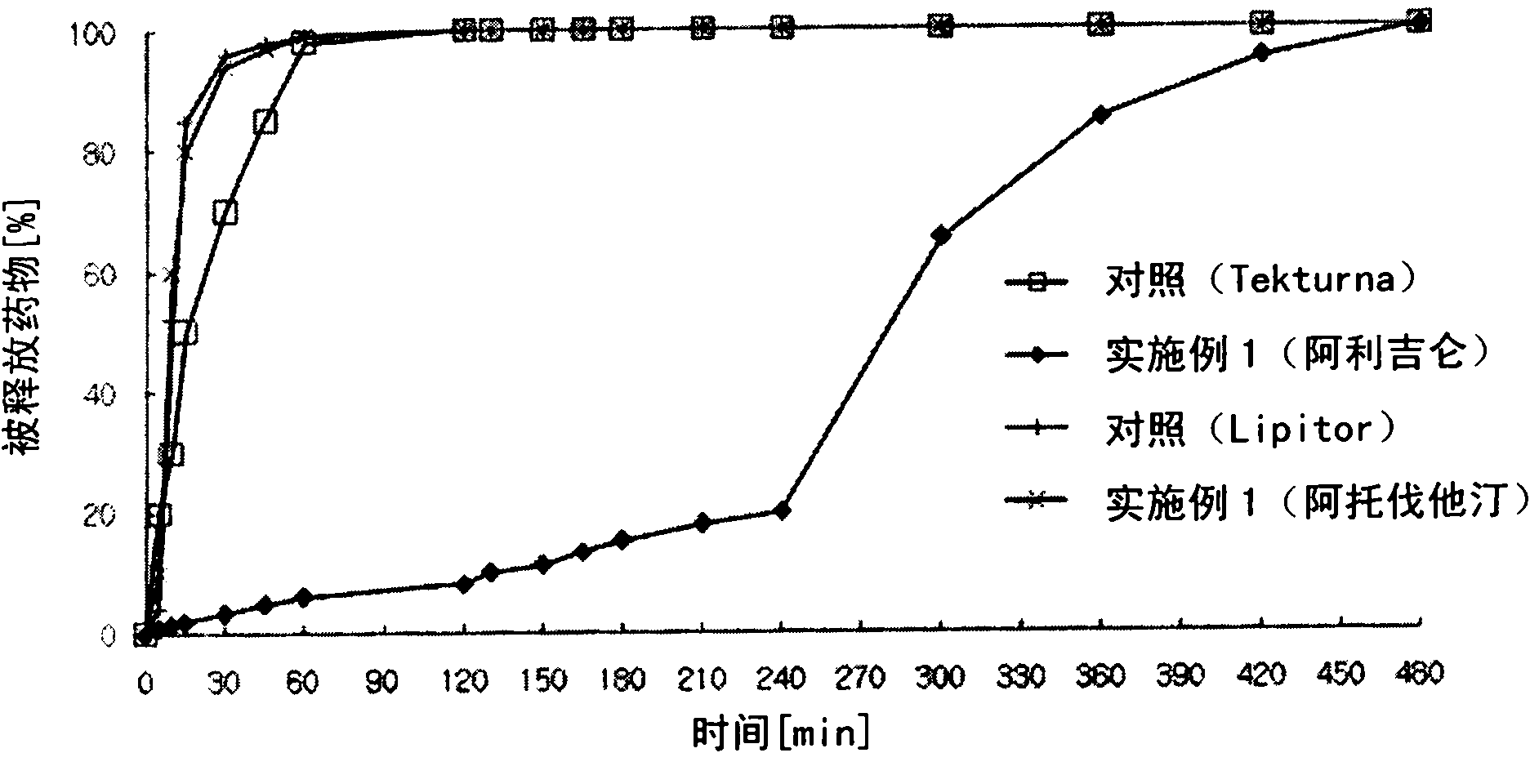
[0143] Example 1: Preparation of Aliskiren-atorvastatin biphasic matrix tablet
[0144] According to the ingredient composition and content shown in Table 1 below, the preparation was carried out as follows.
[0145] 1) Preparation of Aliskiren sustained-release granules
[0146] Aliskiren hemifumarate, microcrystalline cellulose, crospovidone and sodium chloride were sieved through a No. 35 sieve and mixed in a high speed mixer for 5 minutes to prepare a mixture. Meanwhile, polyvinylpyrrolidone was dissolved in purified water to prepare a binding solution, followed by kneading, granulation, and drying. Place the dried material in a fluid bed coater. Meanwhile, cellulose acetate (acetyl 32%), cellulose acetate (acetyl 39.8%), and hydroxypropylmethylcellulose were dissolved and dispersed in 220 mg of ethanol and 980 mg of dichloromethane to prepare a coating solution , which was then coated on the granules in a fluidized bed coater (GPCG-1: Glatt, Germany) to prepare ali...
Embodiment 2
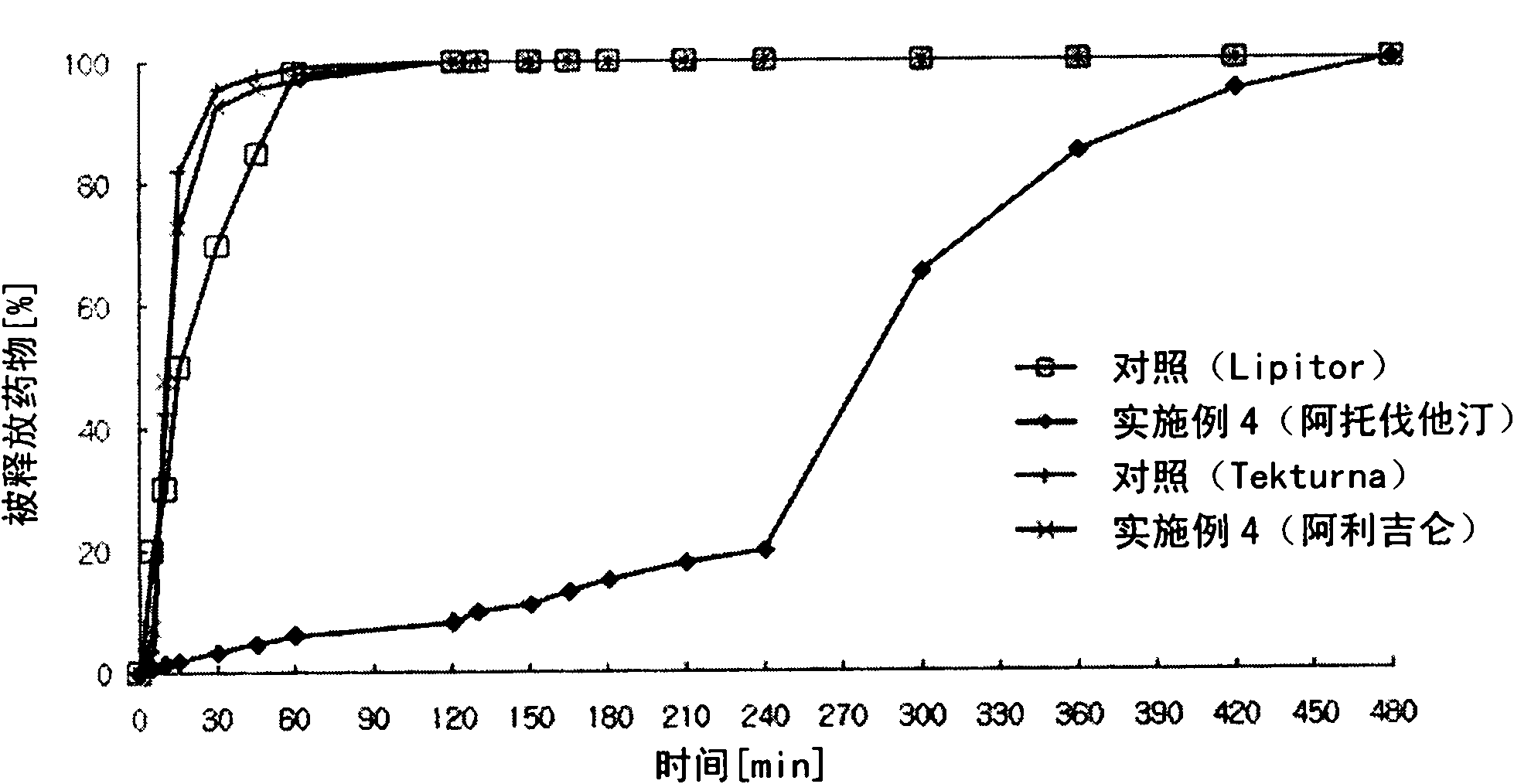
[0151] Embodiment 2: Preparation of Aliskiren-simvastatin biphasic matrix tablet
[0152] According to the ingredient composition and content shown in Table 1 below, the preparation was carried out as follows.
[0153] 1) Preparation of Aliskiren sustained-release granules
[0154] Aliskiren hemifumarate and microcrystalline cellulose were sieved through a No. 35 sieve and mixed in a high-speed mixer for 5 minutes to prepare a mixture. Meanwhile, polyvinylpyrrolidone was dissolved in purified water to prepare a binding solution, which was then kneaded, granulated, and dried. Place the dried material in a fluid bed coater. At the same time, add and then, dissolve and disperse hydroxypropyl methylcellulose phthalate and hydroxypropyl methylcellulose in 220 mg of ethanol and 980 mg of dichloromethane to prepare a coating solution, and then the coating The solution was coated on the granules in a fluidized bed coater (GPCG-1: Glatt, Germany) to prepare aliskiren hemifumarate ...
Embodiment 3
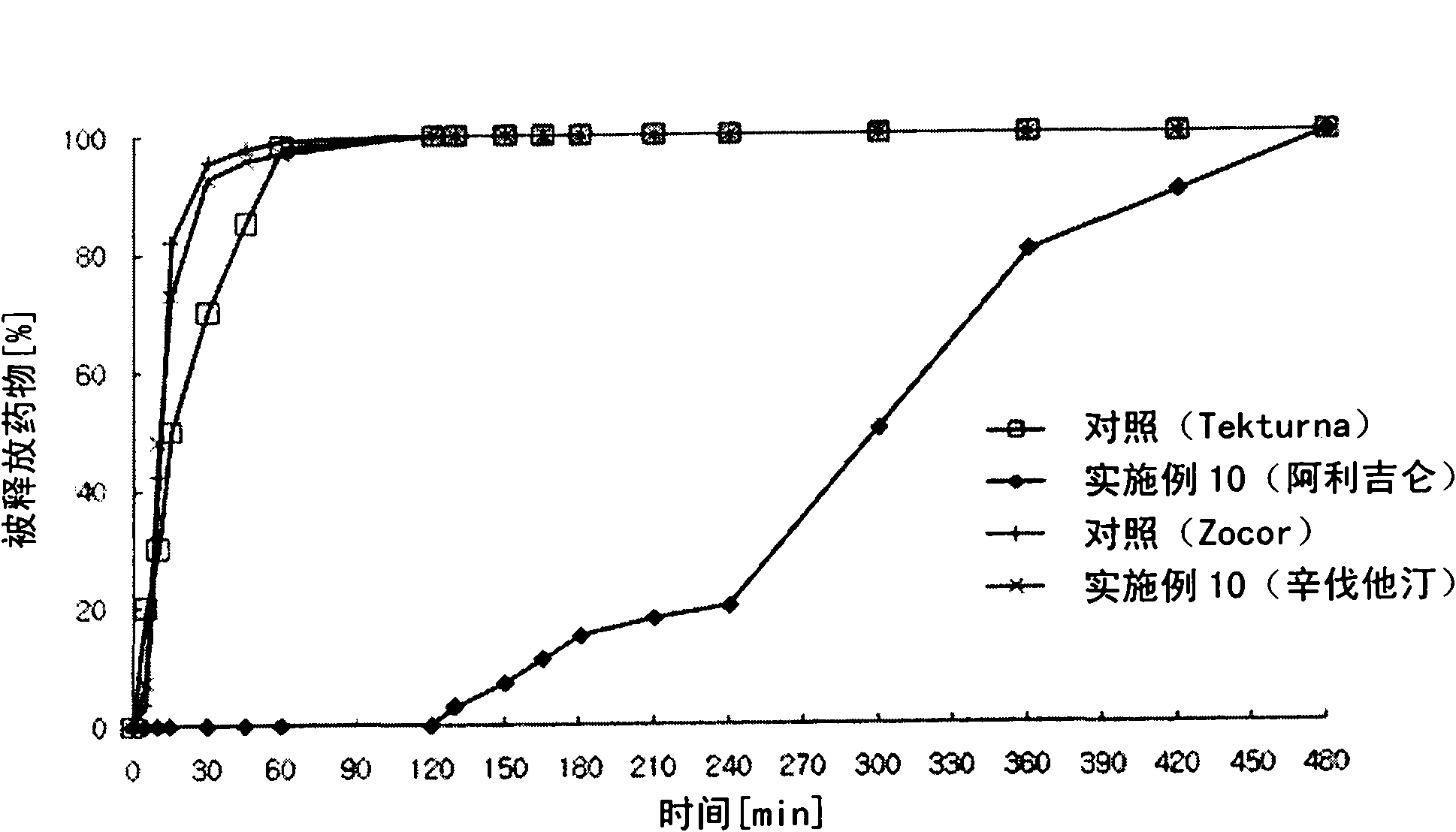
[0159] Example 3: Preparation of Aliskiren-fluvastatin biphasic matrix tablet
[0160] According to the ingredient composition and content shown in Table 1 below, the preparation was carried out as follows.
[0161] 1) Preparation of Aliskiren sustained-release granules
[0162] Aliskiren hemifumarate and microcrystalline cellulose were sieved through a No. 35 sieve and mixed in a high speed mixer for 5 minutes to prepare a mixture. Meanwhile, polyvinylpyrrolidone was dissolved in purified water to prepare a binding solution, followed by kneading granulation and drying. Place the dried material in a fluid bed coater. Meanwhile, Kollicoat SR 30D was dissolved and dispersed in 220 mg of ethanol and 980 mg of dichloromethane to prepare a coating solution, which was then coated in a fluidized bed coater (GPCG-1: Glatt, Germany). Granules to prepare aliskiren hemifumarate sustained-release granules.
[0163] 2) Preparation of fluvastatin prior-release granules
[0164] F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com