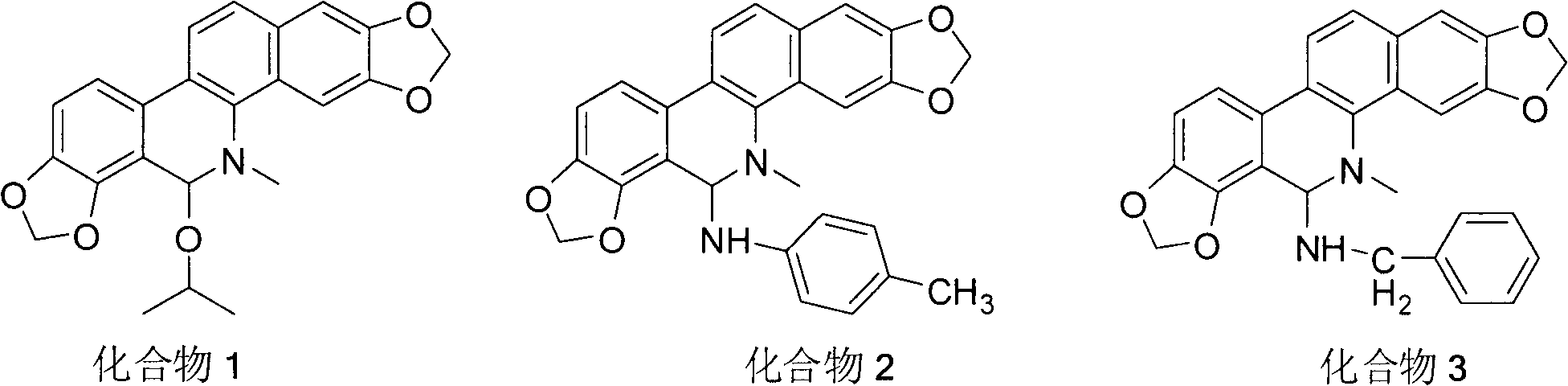
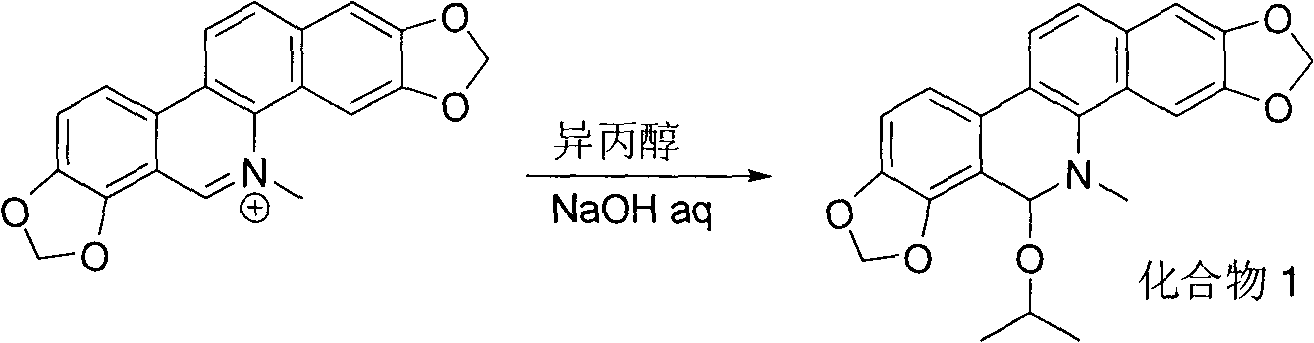
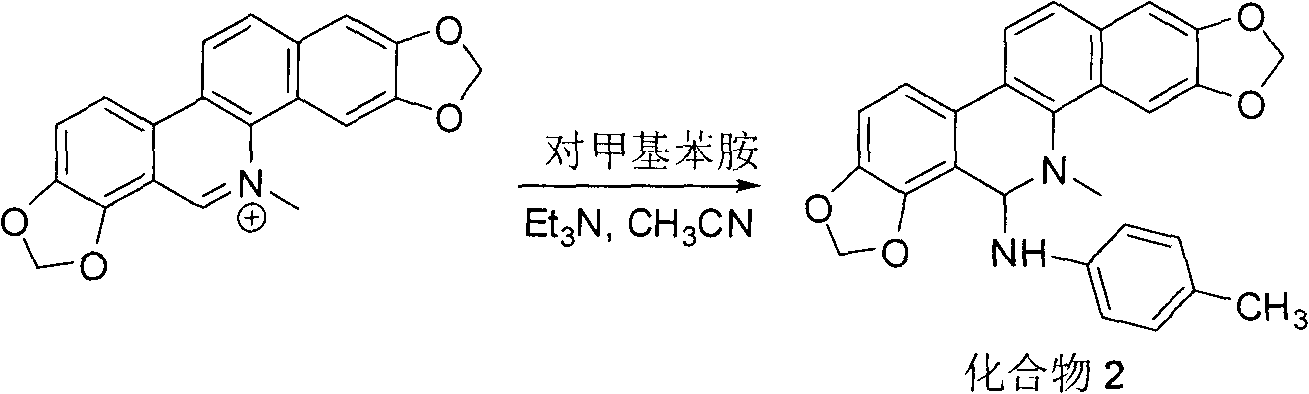
Sanguinarine derivatives and synthesis method and use thereof
A synthetic method and technology of sanguinarine, applied in the field of sanguinarine derivatives and synthesis, can solve problems such as sheath blight, achieve low residue, simple synthesis method, and excellent effect of inhibiting rice sheath blight activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] Compound 1: 110 mg of sanguinarine was dissolved in 5 ml of isopropanol, 5 ml of 0.1 N NaOH solution was added, and stirred at room temperature for 24 hours. Phenomenon: The orange yellow gradually disappears, and a white solid appears. Suction filtration and washing with water gave the product with a yield of 65%. Mp: 247-250°C. 1 H NMR (CDCl 3 , 400MHz) (δ, ppm): 7.76(d, J=8.4Hz, 1H), 7.65(s, 1H), 7.47(d, J=8.4Hz, 1H), 7.39(d, J=8Hz, 1H) , 7.12(s, 1H), 6.91(d, J=8.4Hz, 1H), 6.11(s, 1H), 6.06(s, 3H), 5.53(s, 1H), 4.33(quint, J 1 =6.4Hz,J 2 =6Hz), 2.73(s, 3H), 1.28(d, J=6Hz, 1H), 0.90(d, J=6.4Hz, 1H). 13 C NMR (CDCl 3 , 100MHz) (δ, ppm): 148.0, 147.3, 145.1, 138.7, 130.9, 126.9, 125.9, 123.6, 123.0, 120.3, 116.4, 113.6, 108.6, 104.6, 101.7, 101.0, 100.6, 82.3, 46.8, 2, 3 , 21.2. HRMS (ESI): m / z (%) calcd for [C 23 h 21 NO 5 +Na] + : 414.1312; found: 414.1316.
[0017] Compound 2: 110 mg of sanguinarine in 10 ml of acetonitrile, 0.1 g of p-methylaniline, stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com