Method for preparing bismuth oxychloride by solid-phase reaction at room temperature
A room temperature solid-phase reaction, bismuth oxychloride technology, applied in chemical instruments and methods, inorganic chemistry, bismuth compounds, etc., can solve problems such as low temperature, and achieve the effect of simple process, broad market prospects, and easy amplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
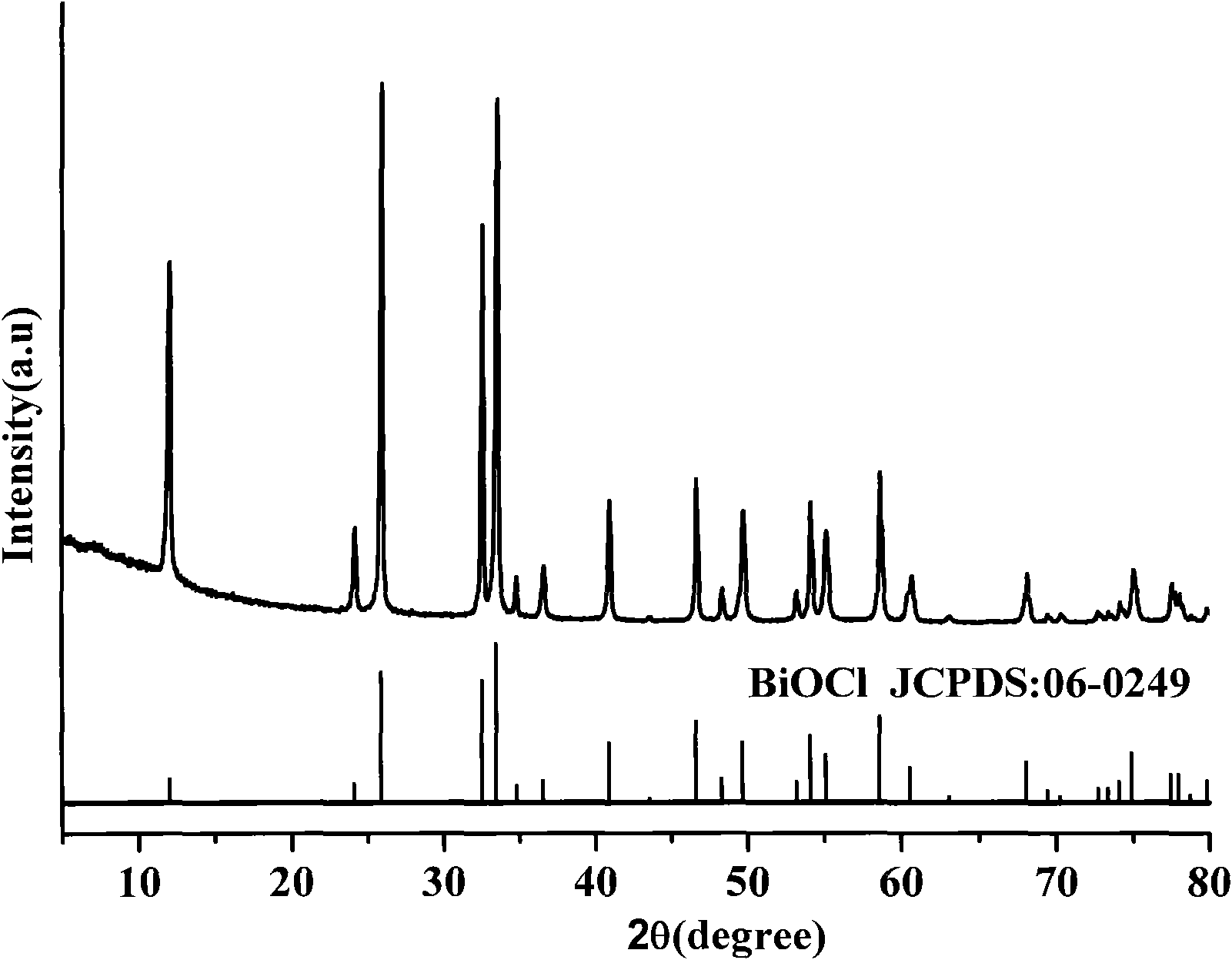
[0019] Weigh bismuth nitrate and sodium chloride by molar ratio Bi:Cl=1:1, mix and grind at room temperature for 0.5 hour;
[0020] The reactants undergo a solid-phase reaction at room temperature, from powder raw materials to porridge paste reactants, and the reaction ends after 30 minutes to obtain powder products;
[0021] Wash the powder with water until the soluble by-product sodium nitrate is removed, and dry to obtain the bismuth oxychloride product, and the reaction yield is 89.7% after weighing.
Embodiment 2
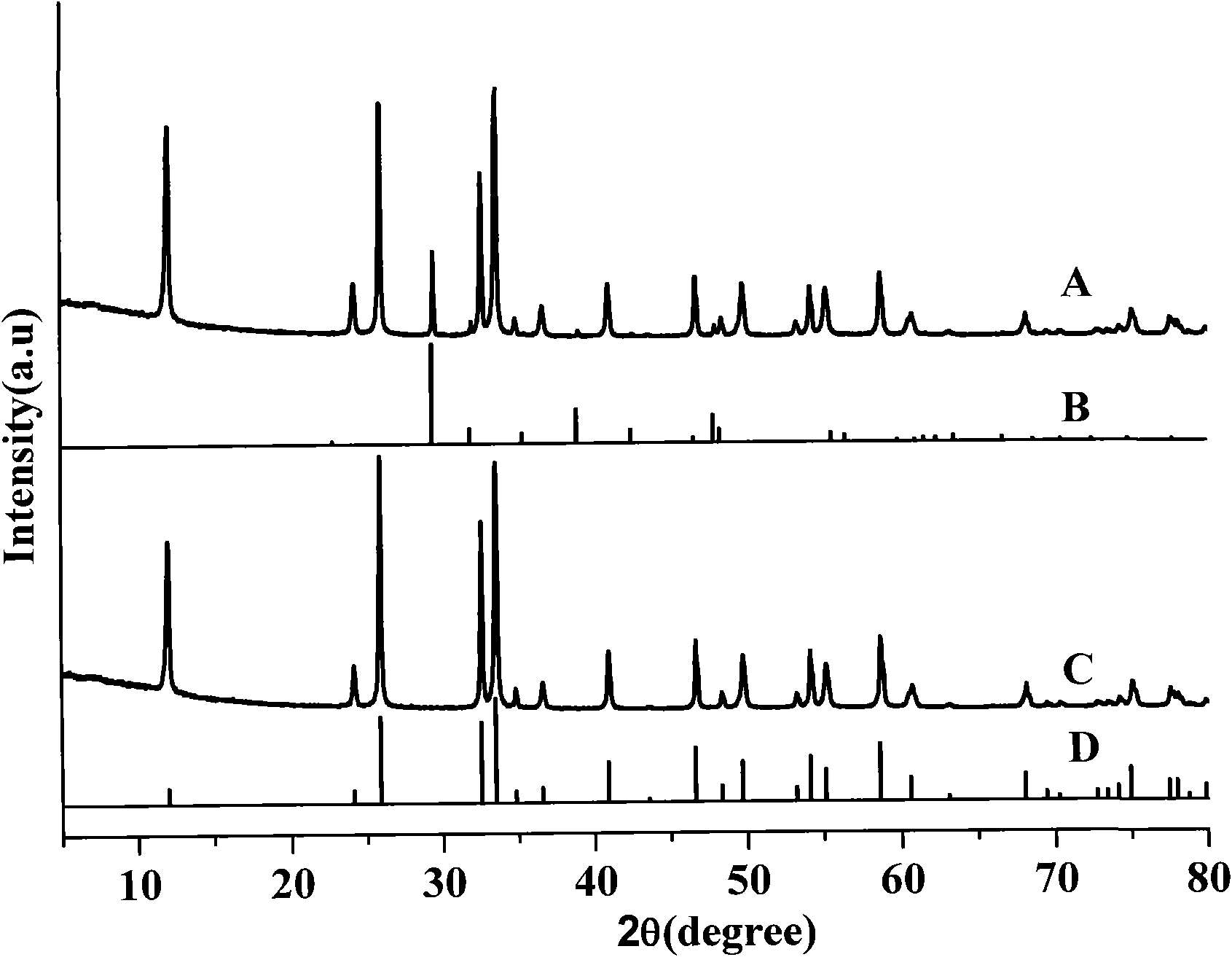
[0023] Weigh bismuth carbonate and sodium chloride at a molar ratio of Bi:Cl=1:2, mix and grind at room temperature for 1 hour;
[0024] The reactants undergo a solid-phase reaction at room temperature, from powder raw materials to porridge paste reactants, and the reaction ends after 30 minutes to obtain powder products;
[0025] The powder is washed with water until the soluble by-product sodium carbonate is removed, and dried to obtain the bismuth oxychloride product, and the reaction yield by weighing is 90.8%.
Embodiment 3
[0027] Weigh bismuth oxalate and sodium chloride at a molar ratio of Bi:Cl=1:1.5, mix and grind at room temperature for 1.5 hours;
[0028] The reactants undergo a solid-phase reaction at room temperature, from powder raw materials to porridge paste reactants, and the reaction ends after 30 minutes to obtain powder products;
[0029] The powder was washed with water until the soluble by-product sodium oxalate was removed, and then dried to obtain the bismuth oxychloride product, and the reaction yield was 85.9% after weighing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com