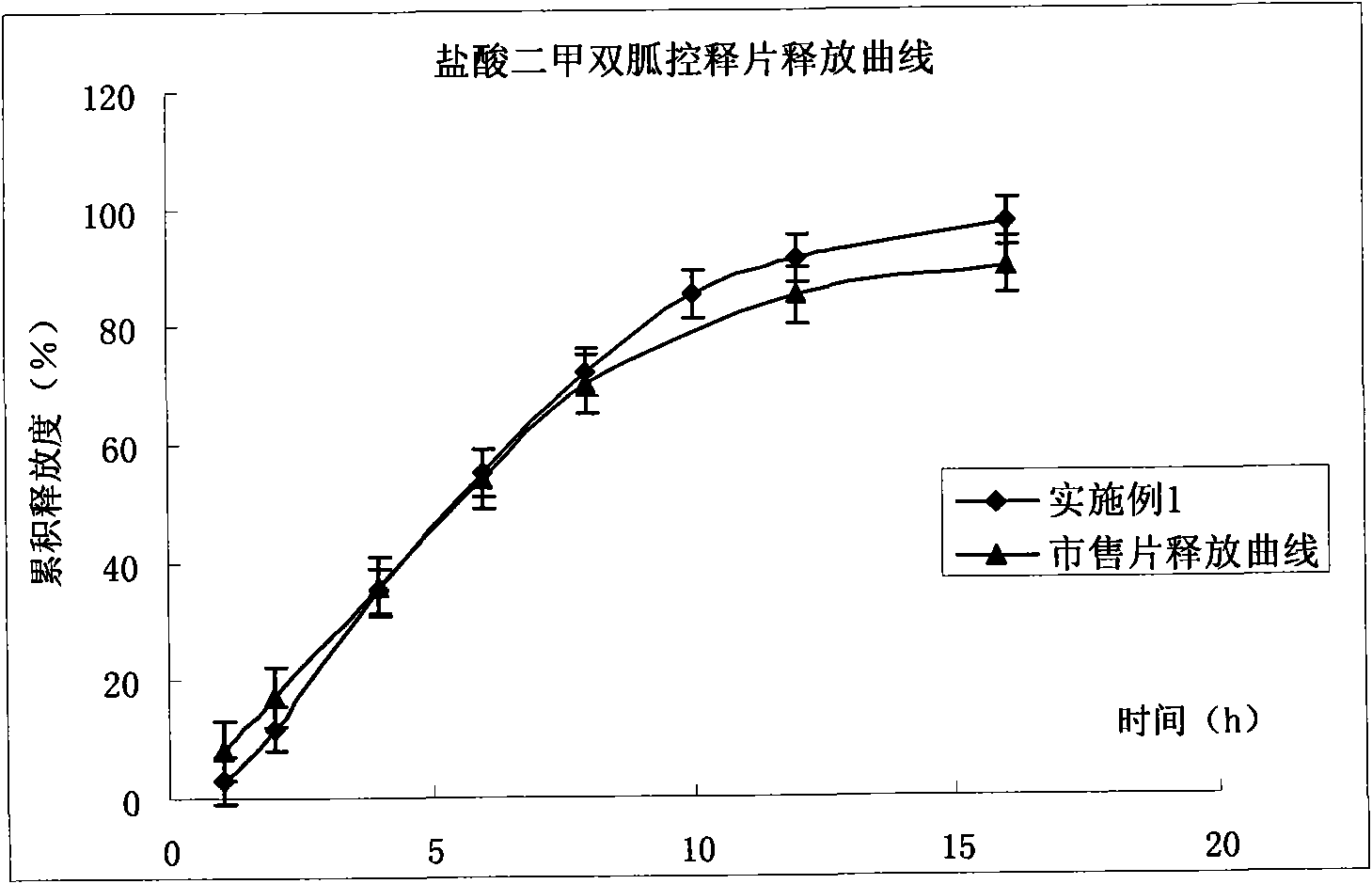
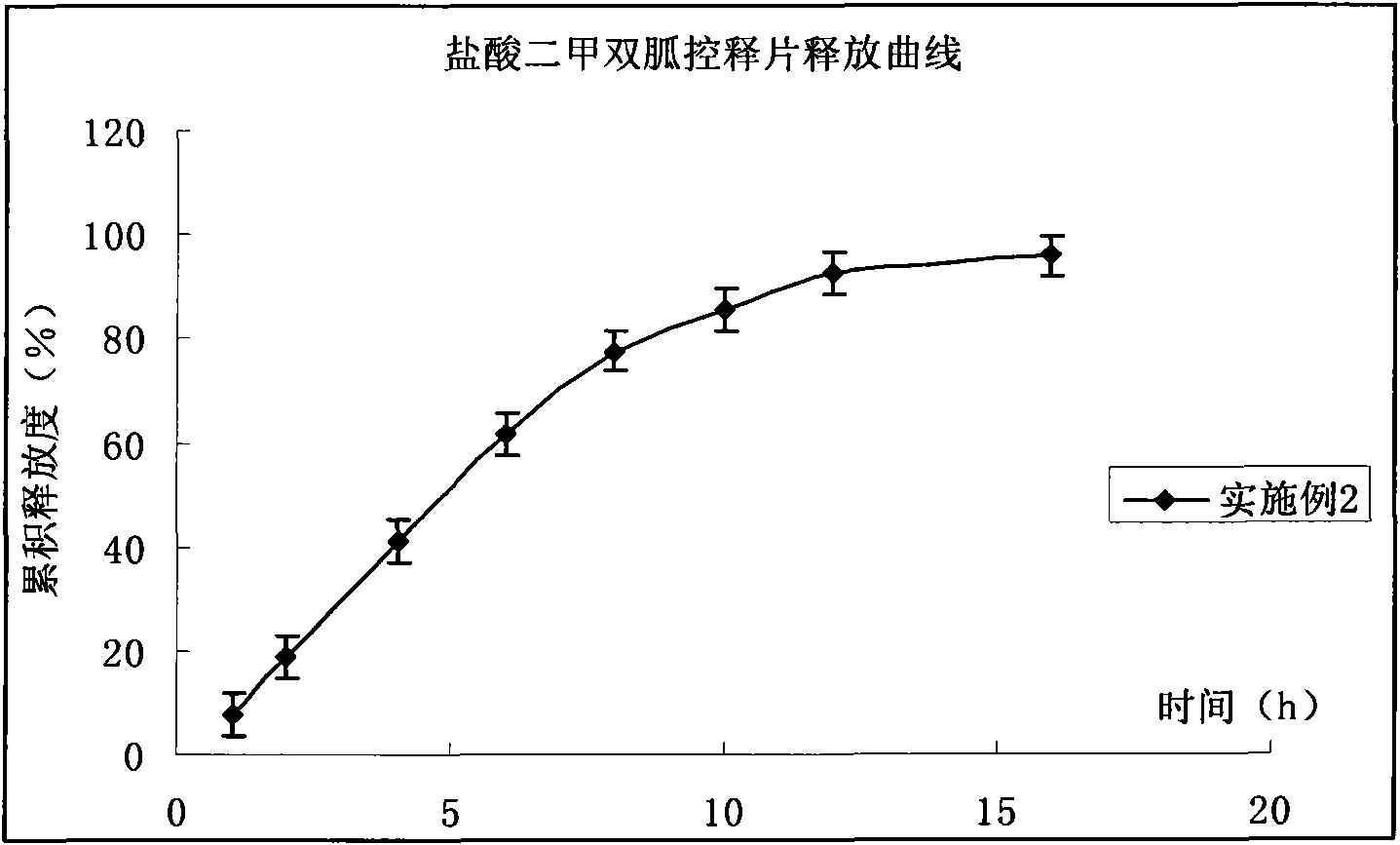
Compound pioglitazone hydrochloride/metformin hydrochloride bilayer osmotic pump controlled release preparation and preparation method thereof
A technology of pioglitazone hydrochloride and metformin hydrochloride, which is applied in the field of medicine, can solve the problems of poor thermal stability of polyoxyethylene, poor thermoplasticity of polyoxyethylene, and affecting the release of controlled-release tablets, so as to reduce residual amount, optimize release, and reduce shear force Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] 1. Prescription
[0092] (1) Composition of drug-containing layer (for every 1000 tablets)
[0093] Name Dosage
[0094] Metformin Hydrochloride 500g
[0095] Polyoxyethylene (303) 40g
[0096] Povidone K30 10g
[0097] Sodium Lauryl Sulfate 8g
[0099] (2) Booster layer composition (for every 1000 pieces)
[0100] Name Dosage
[0101] Polyoxyethylene 50g
[0102] Sodium Alginate 50g
[0104] Povidone K30 20g
[0105] Iron Oxide Black 1g
[0107] (3) Composition of isolation coat coating solution (for every 1000 tablets)
[0108] Name Dosage
[0109] Hypromellose (E5) 15g
[0110] Polyethylene glycol 5g
[0111] water 50ml
[0112] 95% ethanol 400ml
[0113] (4) Composition of coating solution for controlled release coating (for every 1000 tablets)
[0114] Name Dosage
[0115] Cellulose acetate 40g
[0116] Povidone K30 20g
[0117] Acetone 1000ml
[0118] ...
Embodiment 2
[0162] prescription:
[0163] (1) Composition of drug-containing layer (for every 1000 tablets)
[0164] Name Dosage
[0165] Metformin Hydrochloride 1000g
[0166] Polyoxyethylene (301) 60g
[0167] Sodium Lauryl Sulfate 16g
[0168] Povidone K30 20g
[0170] (2) Booster layer composition (for every 1000 pieces)
[0171] Name Dosage
[0172] Polyoxyethylene 80g
[0173] Sodium Alginate 120g
[0174] Sodium chloride 100g
[0175] Povidone K30 46g
[0176] Iron Oxide Black 2g
[0177] Magnesium Stearate 2g
[0178] (3) Composition of isolation coat coating solution (for every 1000 tablets)
[0179] Name Dosage
[0180] Hypromellose (E5) 30g
[0181] Polyethylene glycol 10g
[0182] water 100ml
[0183] 95% ethanol 800ml
[0184] (4) Composition of coating solution for controlled release coating (for every 1000 tablets)
[0185] Name Dosage
[0186] Cellulose acetate 80g
[0187] Povidone K30 40g
[0188] Acetone 2000ml
[01...
Embodiment 3
[0206] prescription:
[0207] (1) Composition of drug-containing layer (dosage per 1000 tablets)
[0208] Name Dosage
[0209] Metformin Hydrochloride 750g
[0210] Polyoxyethylene (303) 50g
[0211] Povidone (K90) 25g
[0212] Sodium caprate 3g
[0213] Magnesium Stearate 2g
[0214] (2) Booster layer composition (for every 1000 pieces)
[0215] Name Dosage
[0216] Polyoxyethylene 50g
[0217] Sodium Alginate 80g
[0218] Sodium chloride 75g
[0219] Povidone (K90) 26g
[0220] Iron Oxide Black 2g
[0221] Magnesium Stearate 2g
[0222] (3) Composition of isolation coat coating solution (for every 1000 tablets)
[0223] Name Dosage
[0224] Hypromellose (E5) 20g
[0225] Polyethylene glycol 4g
[0226] water 75ml
[0227] 95% ethanol 600ml
[0228] (4) Composition of coating solution for controlled release coating (for every 1000 tablets)
[0229] Name Dosage
[0230] Cellulose acetate 60g
[0231] Povidone (K30) 25g
[0232] Acetone 1500ml
[0233] 95% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com