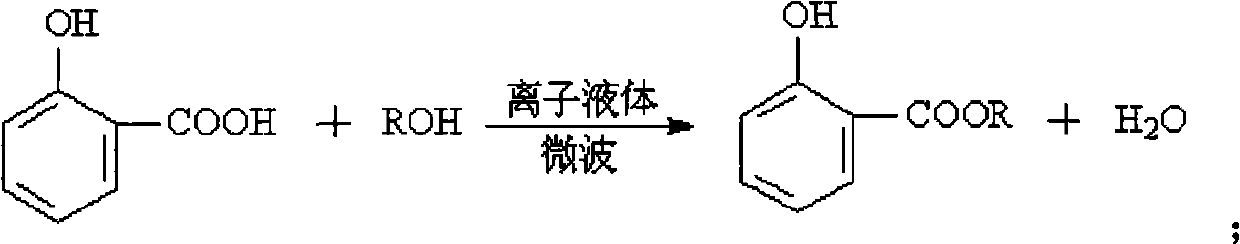
Method for catalytic synthesis of salicylate alkyl ester by using ionic liquid under microwave promotion
An alkyl salicylate and ionic liquid technology, applied in the field of organic compound preparation, can solve the problems of complex process, unfriendly environment, long reaction time, etc., and achieve the realization of catalytic process, convenient separation and recovery, high catalytic activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
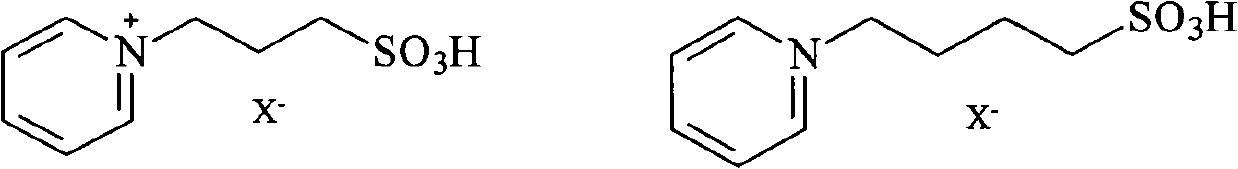
[0027] Example 1: Add 0.02mol salicylic acid and 0.06mol methanol (the molar ratio of salicylic acid to methanol is 1:3) into a microwave-specific reaction vessel, add 10mmol of ionic liquid [PSPy][HSO 4 ], stirred and dissolved, and placed in a microwave-assisted synthesizer to react; set the reaction temperature to 105°C, the rated power to 1360W, and the radiation time to 20min. After the reaction, the upper layer of the system is methyl salicylate, and the product and unreacted salicylic acid can be separated from the system by ether extraction. The conversion rate of salicylic acid was 93.7%, and the selectivity of methyl salicylate was 99.9%. The ionic liquid in the lower layer was dried in a blast drying oven at 90°C for 6 hours, and then the substrate was added, and the reaction could be repeated 8 times as above. The reaction results are shown in Table 1.
[0028] Table 1 The relationship between catalyst use times and catalytic activity
[0029]
example 2
[0030] In Example 2, except that the molar ratio of salicylic acid and methanol in the raw material was changed to 1:2, other experimental conditions and ionic liquid were the same as in Example 1 to prepare methyl salicylate. The conversion rate of salicylic acid was 81.3%, and the selectivity of methyl salicylate was 99.9%.
example 3
[0031] In Example 3, except that the amount of the ionic liquid in the reaction condition was changed to 8 mmol, other experimental conditions and ionic liquid were the same as in Example 1, and methyl salicylate was prepared. The conversion rate of salicylic acid was 82.2%, and the selectivity of methyl salicylate was 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com