Mesoporous composite oxide type solid super acidic catalyst and preparation method thereof
A composite oxide, solid superacid technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve superacid deactivation, service life and repetition Problems such as poor usability, production and application, to achieve the effects of uniform grain size, excellent catalytic activity, excellent catalytic activity and reusability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
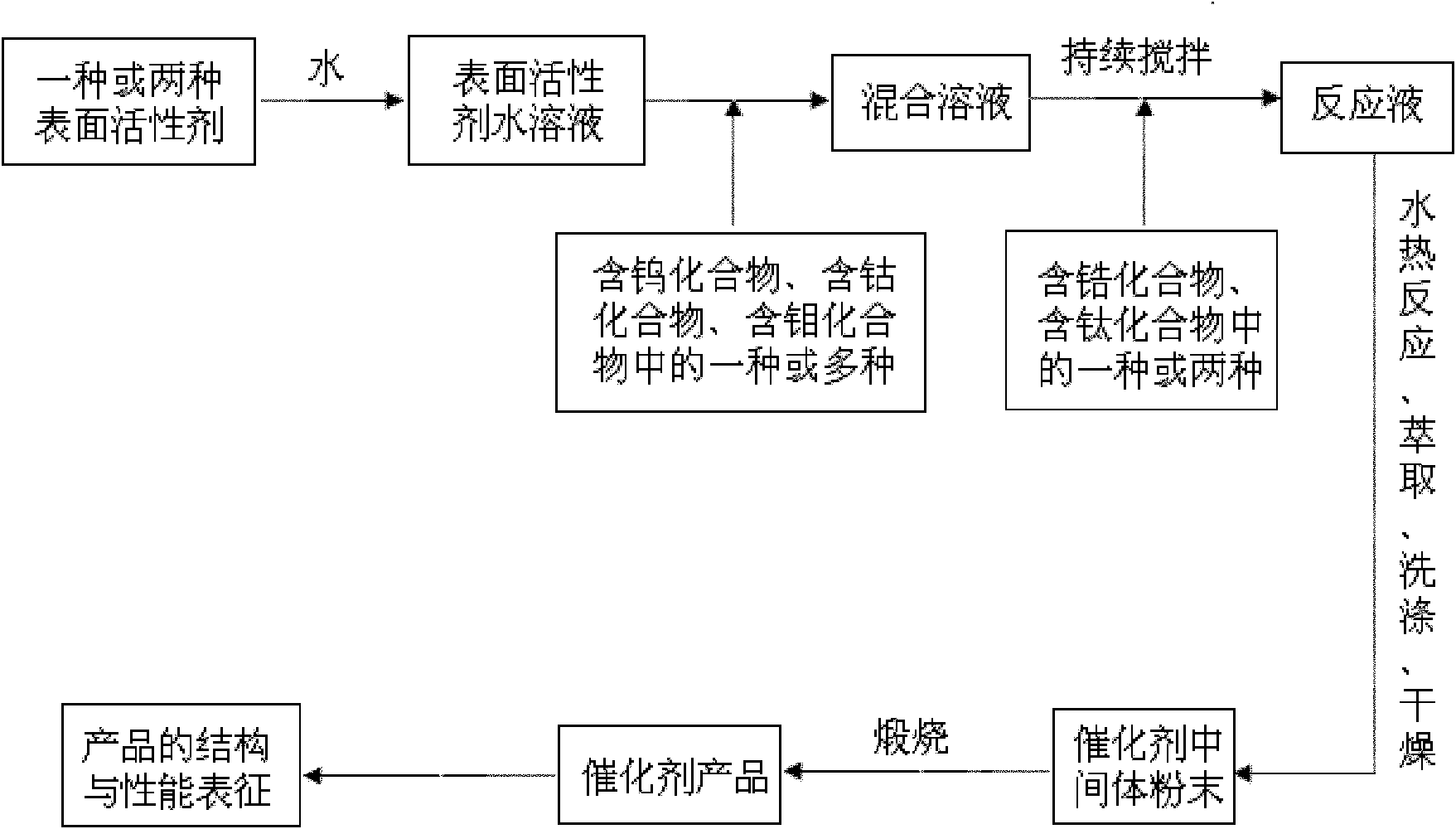
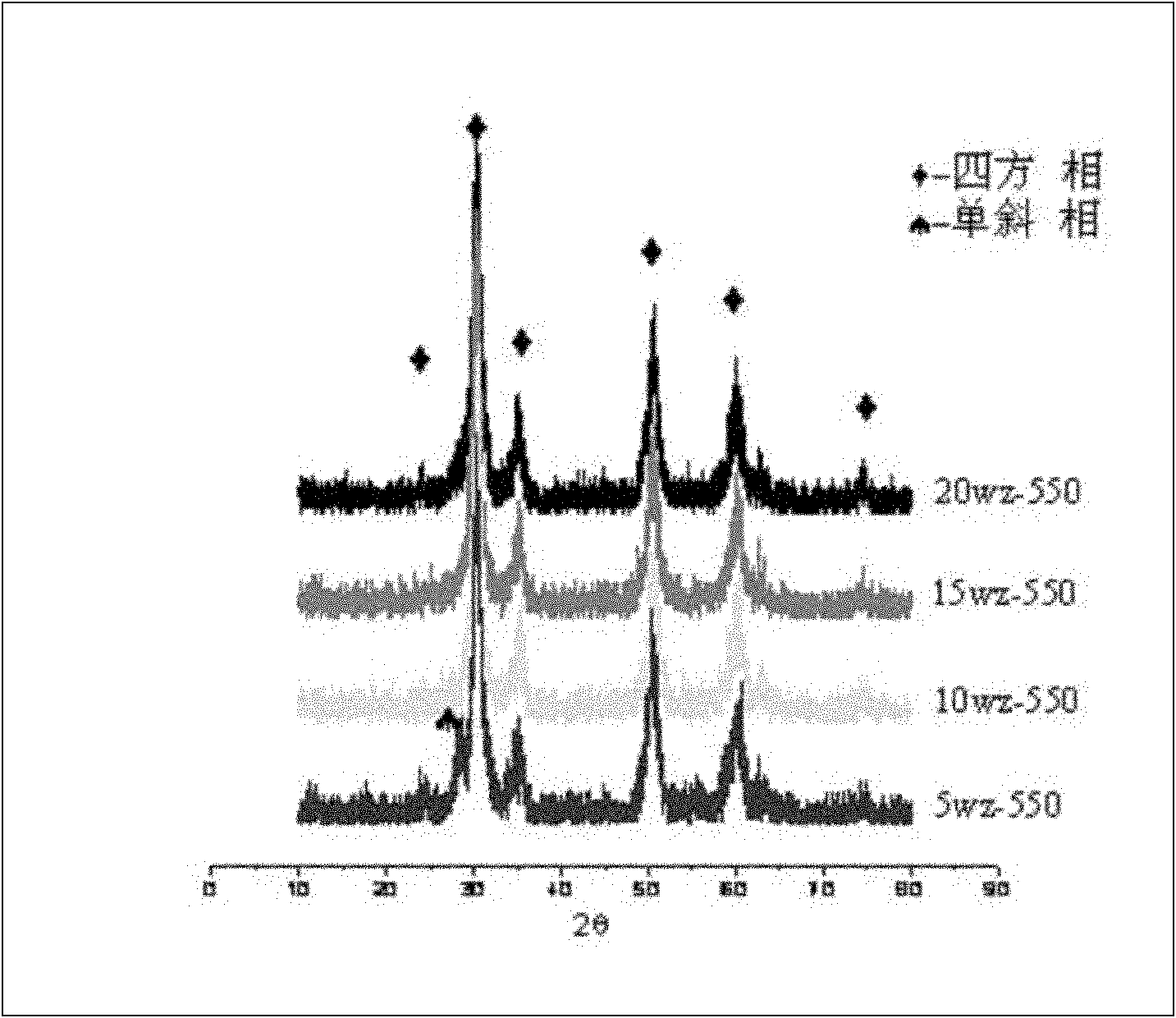
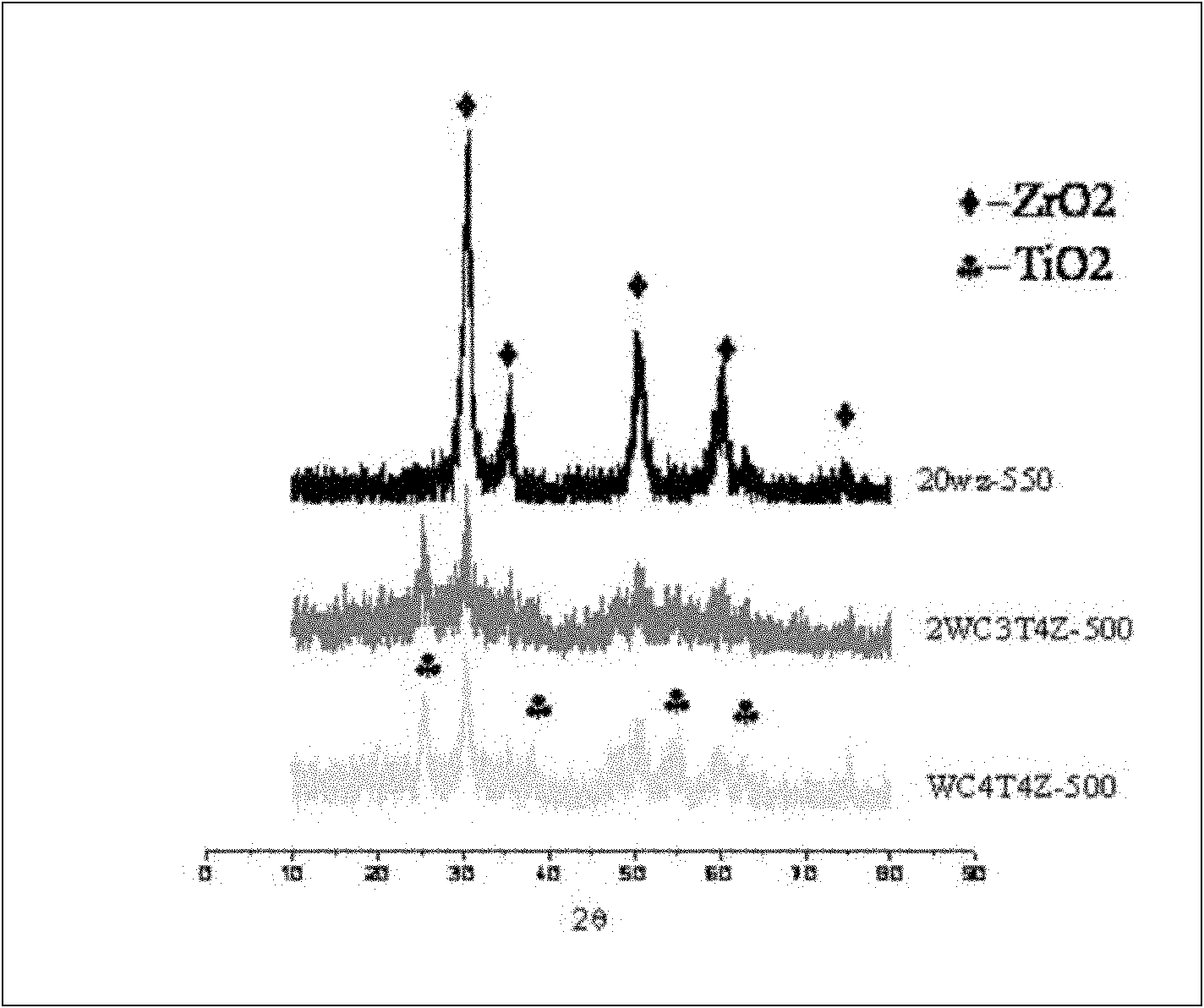
[0038] Weigh 3.7g and 4.5g of the two template agents P123 and Brij35, respectively, and dissolve them in 100ml of aqueous solution. When stirring until the solution is clear, first add 0.7g of phosphotungstic acid to dissolve, so that the phosphotungstic acid is completely dissolved in the above solution, then add 10ml of zirconium propoxide (70%wt) drop by drop, and stir for 12h. The samples were placed in an oven at 100°C for 24 hours. After the hydrothermal treatment, the sample was taken out, extracted with ethanol solution to remove the surfactant, and then placed in an oven at 100°C to dry the sample for 24 hours. After the sample was dried, it was placed in a muffle furnace and calcined at a high temperature of 550° C. for 5 hours to obtain a composite oxide solid acid catalyst. The mass ratio of each component in the composite oxide solid acid catalyst of this embodiment is: tungsten oxide 20%wt, zirconium oxide 80%wt. Its typical XRD diffraction pattern is as fig...
Embodiment 2
[0044] According to the preparation process of Example 1, the sample was dried and put into a muffle furnace for calcination at a high temperature of 700° C. for 3 hours. The results of the N2-adsorption-desorption analysis are shown in Table 1, and the results of the probe reaction experiment in the esterification process are shown in Table 2. It can be seen that the composite catalytic powder material is still a stable tetragonal phase nanocrystal after being calcined at 700 °C; it has a high specific surface area, and the specific surface area is greater than 90m 2 / g. The catalytic activity of the material is lower than that of the sample calcined at 550°C, and the esterification reaction effect is also relatively low. After 6 hours of reaction, the esterification conversion rate reaches 100%.
Embodiment 3
[0046] According to the preparation process of Example 1, the amount of phosphotungstic acid was changed to 0.52g, and the rest of the process flow remained unchanged. The mass ratio of each component in the composite oxide solid acid catalyst of this embodiment is: tungsten oxide 15%wt, zirconium oxide 85%wt. Its typical XRD pattern is as figure 2 As shown in 15wz-550, the results of N2-adsorption and desorption are shown in Table 2, and the results of the probe reaction experiment in the esterification process are shown in Table 3. It can be seen that the composite catalytic material sample of the present embodiment has a high specific surface area, reaching 169m 2 / g, has good catalytic activity, after 1 hour of esterification reaction, the esterification conversion rate is 69%, and after 6 hours of reaction, the esterification conversion rate reaches 100%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com