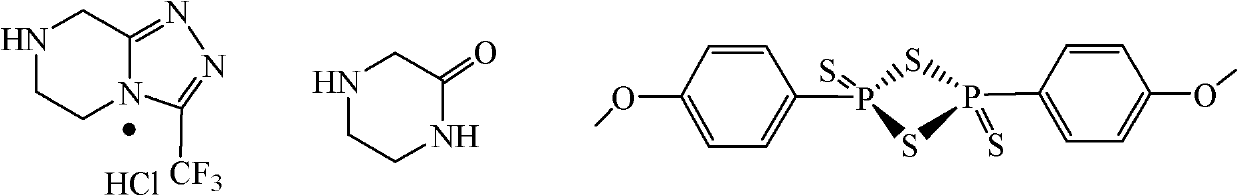
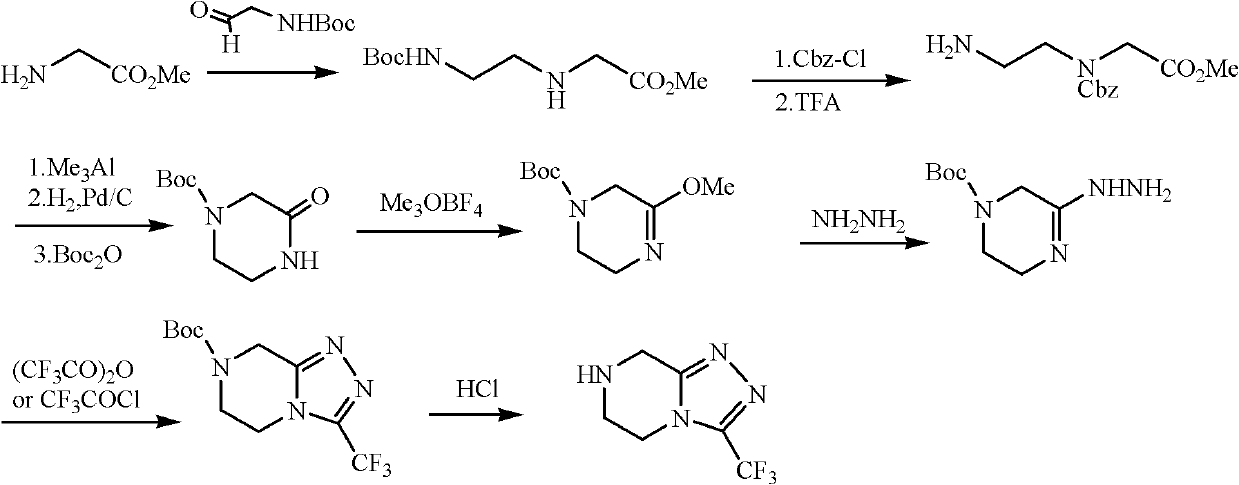
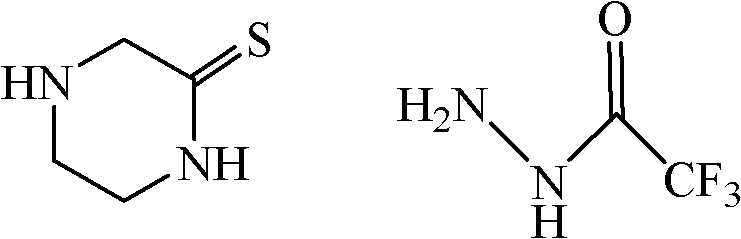Method for preparing sitagliptin phosphate side chain
A technology of hydrochloride and hydrazine trifluoroacetate, which is applied in the field of preparation of side chains of sitagliptin phosphate, can solve problems such as low yield and explosion of anhydrous hydrazine, and achieve high yield, low cost, and cheap raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Under nitrogen protection, to P 2 S 5 (4.3g, 10mmol) in THF (150mL) solution was added 2-piperazinone (1g, 10mmol); electric stirring reaction at room temperature for 2h, then the reaction solution was treated with 30mL 3Mol / L NaOH (aq), and then ethyl acetate Extract the ester, concentrate the organic layer, add 60mL toluene to the crude oil, reflux for 4h, let stand to separate layers, pour out the toluene layer while it is hot, and gradually cool down to room temperature; continue to add 60mL toluene to the remaining oil, and reflux for 3h. Stand to separate layers, pour out the toluene layer while hot, and slowly lower to room temperature. The two toluene phases were mixed and cooled at -4°C for 12 hours, then crystallized, collected by filtration, and washed twice with cold toluene to obtain 0.95 g of a yellow solid, with a yield of 81.7%. The obtained product does not need further purification and can be directly used in the next reaction.
[0036] MS (ESI): m / ...
Embodiment 2
[0038] Under nitrogen protection, the P 2 S 5 (5.2g, 12mmol) and 2-piperazinone (1g, 10mmol) were added into 30mL 1,4-dioxane, and the reaction was performed under electric stirring at room temperature for 3h. Then use 30mL 3Mol / L NaOH (aq) to treat the reaction solution, then extract with ethyl acetate, concentrate the organic layer, add 60mL toluene to the crude oil, reflux for 4h, let stand to separate layers, pour out the toluene layer while hot, gradually Cool down to room temperature; continue to add 60mL of toluene to the remaining oil, reflux for 3 hours, let stand to separate layers, pour out the toluene layer while hot, and slowly cool down to room temperature. The two toluene phases were mixed and cooled at -4°C for 12 hours, then crystallized, collected by filtration, and washed twice with cold toluene to obtain 0.91 g of a yellow solid, with a yield of 78.4%. The obtained product does not need further purification and can be directly used in the next reaction. ...
Embodiment 3
[0040]Under nitrogen protection, LR (2.43, 6 mmol) was dissolved in THF (150 mL), and 2-piperazinone (1 g, 10 mmol) was added under vigorous stirring, and reacted at room temperature for 4 h. Then use 30mL 3Mol / L NaOH (aq) to treat the reaction solution, then extract with ethyl acetate, concentrate the organic layer, add 60mL toluene to the crude oil, reflux for 4h, let stand to separate layers, pour out the toluene layer while hot, gradually Cool down to room temperature; continue to add 60mL of toluene to the remaining oil, reflux for 3 hours, let stand to separate layers, pour out the toluene layer while hot, and slowly cool down to room temperature. The two toluene phases were mixed and cooled at -4°C for 12 hours, then crystallized, collected by filtration, and washed twice with cold toluene to obtain 0.97 g of a yellow solid, with a yield of 83.6%. The obtained product does not need further purification and can be directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com