Method for continuously producing p-menthane hydroperoxide by p-menthane and device thereof
A hydrogen peroxide and production method technology, applied in the direction of peroxide compound preparation, chemical instruments and methods, separation methods, etc., can solve problems such as poor control accuracy, hidden safety hazards, high labor intensity, etc., to ensure safety and stability, solve Safety issues, the effect of eliminating environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: p-menthane prepares hydrogen peroxide p-menthane continuous production device embodiment
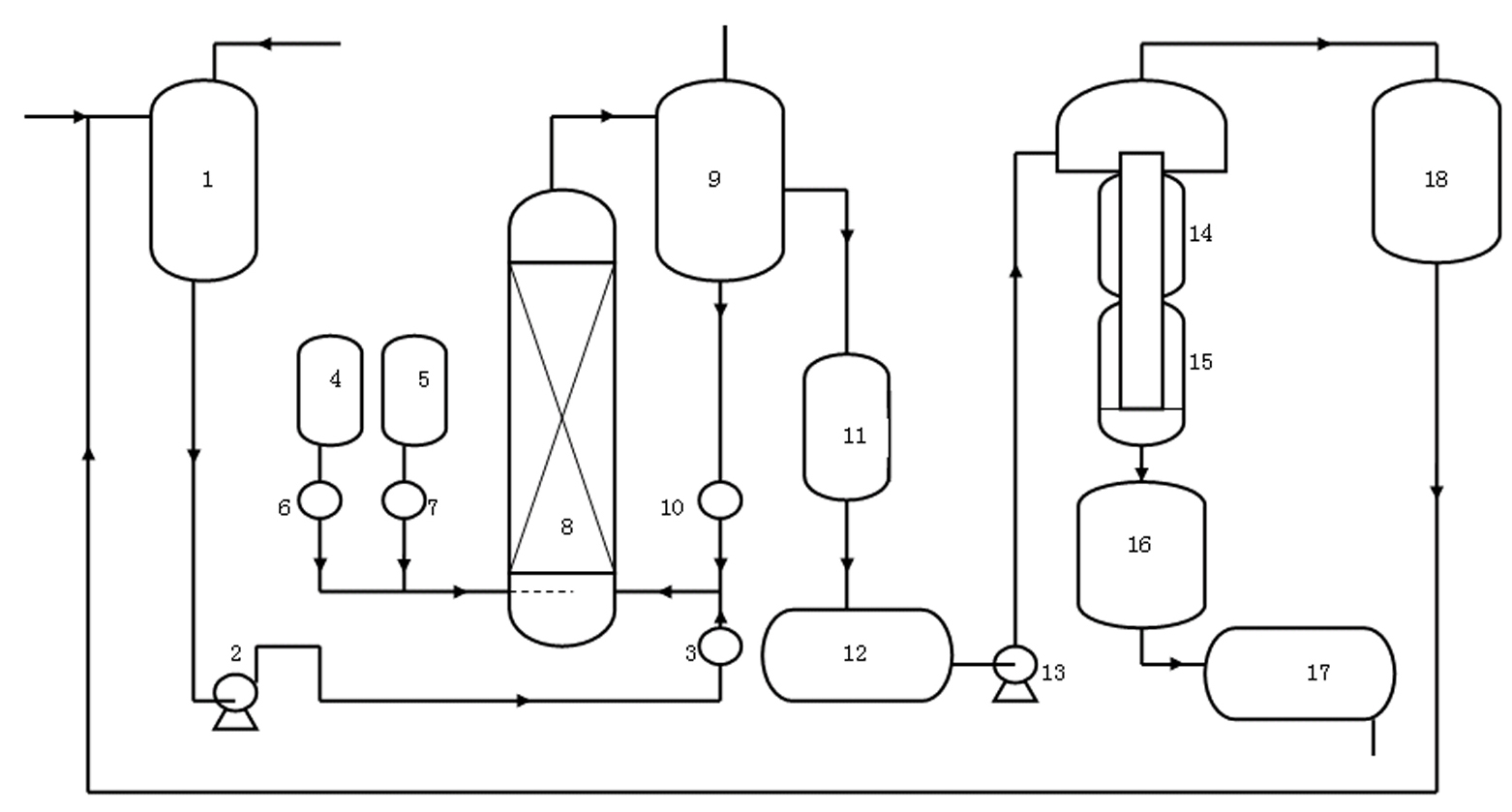
[0031] refer to figure 1 , this embodiment includes raw material mixing tank 1, feeding pump 2, air storage tank 4, oxygen storage tank 5, oxidation tower 8, gas-liquid separator 9, flow meter I3, flow meter II6, flow meter III7, flow meter IV10, Cooler I11 and cooler II16, intermediate storage tank 13, feed pump 13, primary evaporator 14, secondary evaporator 15, product storage tank 17 and condenser 18, outlet of raw material mixing tank 1 and feed pump 2, the feed port of feed pump 2 is connected with the feed port of flow meter I3, the feed port of flow meter I3 is connected with the feed port of the lower part of oxidation tower 8, the outlet of air storage tank 4 and oxygen storage tank 5 The gas ports are respectively connected to the air inlets of flowmeter II6 and flowmeter IV7, and the gas outlets of flowmeter II6 and flowmeter IV7 are connected to the air...
Embodiment 2
[0037] Using catalyst cobalt acetate, ketone acetate or manganese acetate as a free radical initiator, comprising the following specific steps:
[0038] (1) Oxidation reaction: pass air (or oxygen or a mixture of air and oxygen) from air storage tank 4 (or oxygen storage tank 5, or air storage tank 4 and oxygen storage tank 5) through flow meter II6 (or / and flowmeter Ⅲ7) and the air inlet at the lower part of the oxidation tower 8 are blown into the oxidation tower 8, and the jacket is opened for heating. 3 / h ~2.0 m 3 / h flow rate will add the mixed solution that p-menthane and catalyzer are formed in oxidation tower 8 from the bottom of oxidation tower 8, make air fully contact with p-menthane solution, carry out oxidation reaction, control oxidation tower 8 top discharge hydrogen peroxide to The concentration of menthane is 10%~20%; (2) gas-liquid separation: after the oxidation product is discharged from the outlet of the oxidation tower 8, it enters the gas-liquid separa...
Embodiment 3~6
[0042] Step (1) The oxidation reaction temperature is 60°C; the oxygen flow rate is respectively set to 20 m 3 / h, 80m 3 / h, 140m 3 / h, 200m 3 / h, the feed flow rate of Menthane material is 0.5 m 3 / h, 1.0m 3 / h, 1.5 m 3 / h, 2.0m 3 / h; Catalyst cobalt acetate add-on is 0.01%, 0.04%, 0.07%, 0.1% of p-menthane raw material weight; Step (2) hydrogen peroxide is respectively 30 m 3 / h, 50m 3 / h, 70m 3 / h, 100m 3 / h; in step (3), the primary evaporation temperature is controlled at 50°C, the secondary evaporation temperature is controlled at 80°C, and the flow rate of hydrogen peroxide to the menthane product entering the primary evaporator is respectively set to 0.5 m 3 / h, 1.0m 3 / h, 1.5m 3 / h, 2.0m 3 / h, remaining with embodiment 2 。
[0043] Treat that the system is running stably, sampling analysis is respectively 10.2%, 12.5%, 14.8%, 16.7% for the hydrogen peroxide in the discharge at the bottom of the oxidation tower to the Menthane product content, and the hyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com