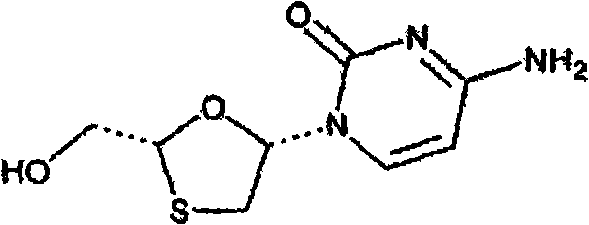
Lamivudine powder and preparation method thereof
A technology of lamivudine and powder, applied in antiviral agents, digestive system, powder delivery, etc., can solve the problem that lamivudine tablets are not suitable for children, the elderly and patients with dysphagia, etc. Fast-acting, easy-to-absorb, fast-dissolution effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
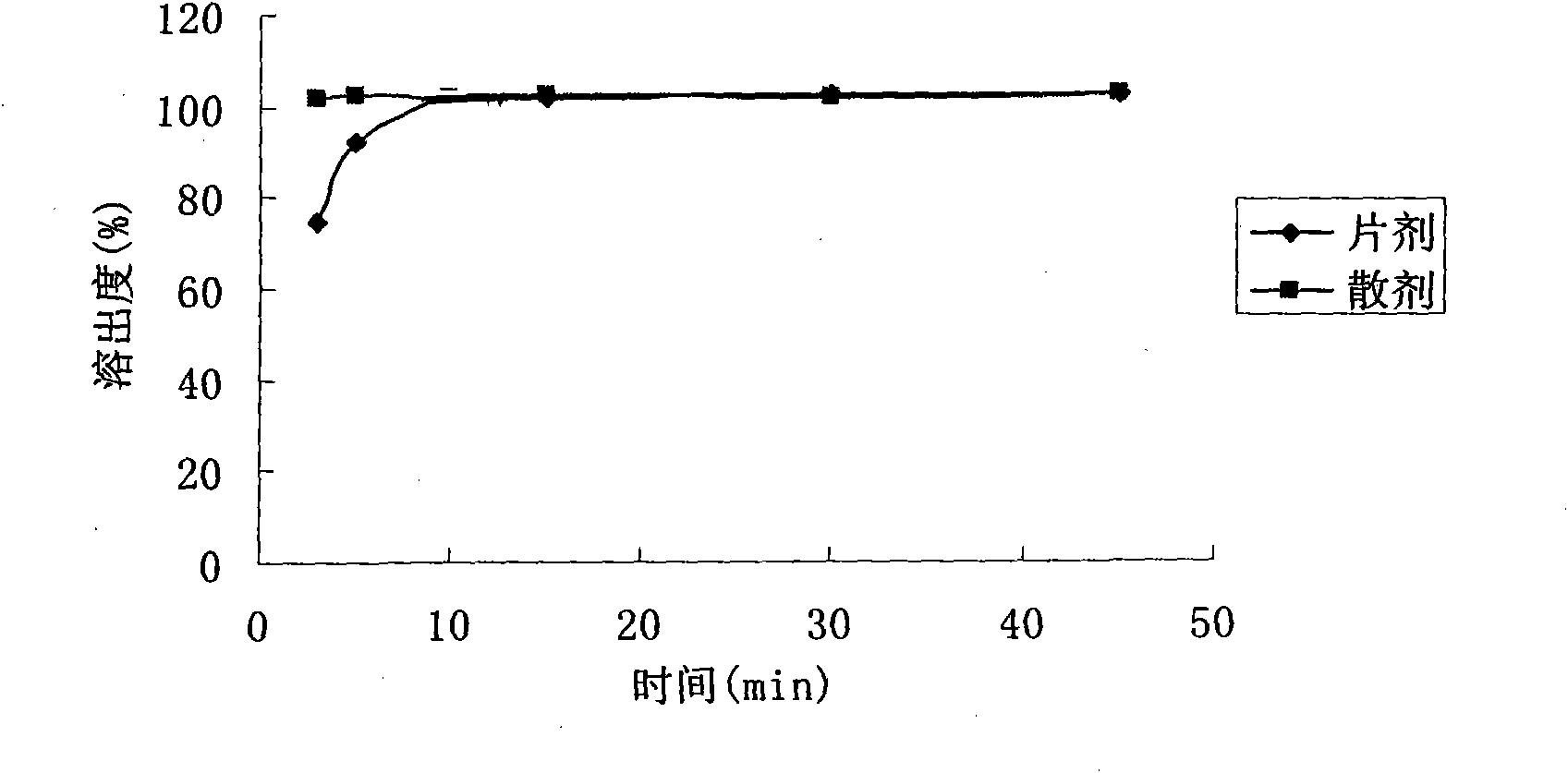
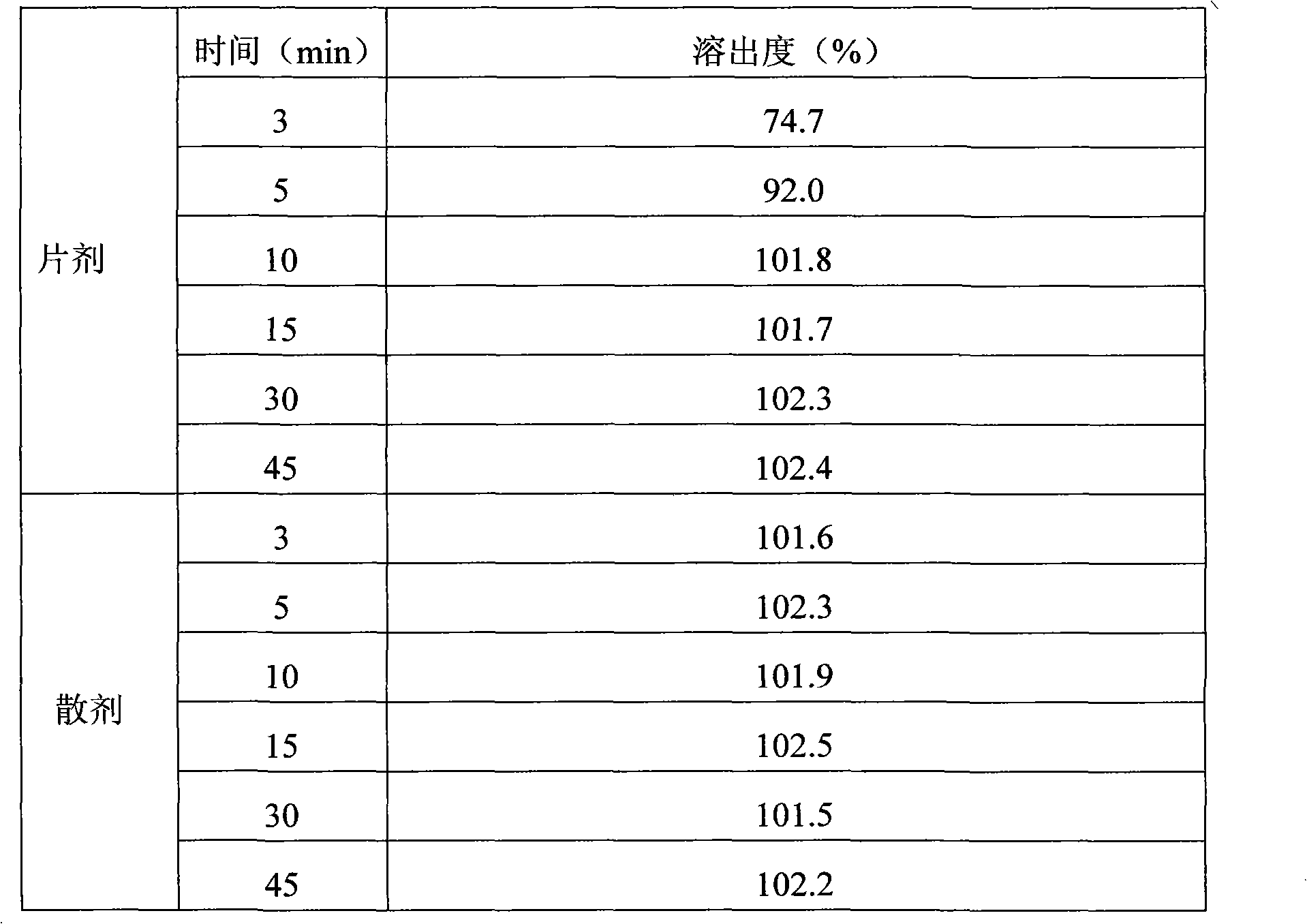
Image
Examples
Embodiment 1
[0039] Raw materials: lamivudine raw material 20g.
[0040] The preparation method of lamivudine powder comprises the following processing steps:
[0041] (1) Take the raw material and dry it until the moisture is 0.4%;
[0042] (2) the raw material after drying is crossed 80 mesh sieves;
[0043] (3) Take medicinal strip-shaped composite packaging film (SP film) as packaging material, fill on SP packaging machine, prepare 1000 bags,
[0044] Each bag contains lamivudine 20mg.
Embodiment 2
[0046] Raw material: 50g of lamivudine raw material.
[0047] The preparation method of lamivudine powder comprises the following processing steps:
[0048] (1) Take the raw material and dry it until the moisture is 0.4%;
[0049] (2) the raw material after drying is crossed 80 mesh sieves;
[0050] (3) Use paper-aluminum composite film as packaging material, fill on a single-row horizontal packaging machine, and prepare 1000 bags, each bag containing 50 mg of lamivudine.
Embodiment 3
[0052] Raw materials: lamivudine raw material 100g.
[0053] The preparation method of lamivudine powder comprises the following processing steps:
[0054](1) Take the raw material and dry it until the moisture is 0.2%;
[0055] (2) the raw material after drying is crossed 80 mesh sieves;
[0056] (3) Take the medicinal strip-shaped composite packaging film (SP film) as the packaging material, fill it on the SP packaging machine, and prepare 1000 bags, each containing 100 mg of lamivudine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com