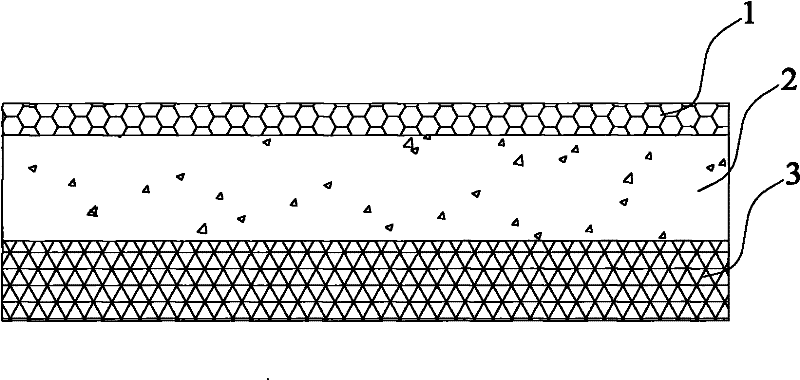
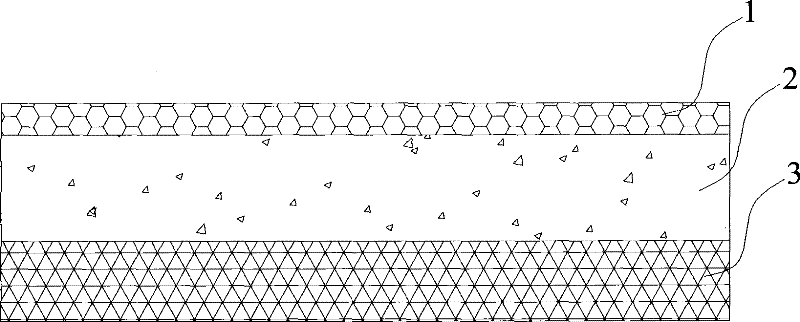
Novel penetration-promoting agent composition and application thereof to transdermal administration system
A technology of transdermal drug delivery system and penetration enhancer, which is applied in the field of pharmaceuticals and can solve problems such as impractical application, viscous or cohesive damage, limited dissolution of levonorgestrel, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Steroid hormones: levonorgestrel 0.05g, ethinyl estradiol 0.05g;
[0082] Viscosity regulator: PVP / VA copolymer (VA64) 0.1g;
[0083] Penetration enhancer 5g: propylene glycol 2.5g, diethylene glycol monoethyl ether 2.5g;
[0084] Adhesive polymer: GMS737 20g
[0085] The measured transdermal rate of levonorgestrel was 0.14 μg / cm 2 / h, ethinyl estradiol transdermal rate: 0.14μg / cm 2 / h.
Embodiment 2
[0087] Steroid hormones: levonorgestrel 0.2g, ethinyl estradiol 0.1g;
[0088] Viscosity modifier: PVP / VA copolymer (VA64) 1.5g;
[0089] Penetration enhancer 6g: propylene glycol 2g, diethylene glycol monoethyl ether 4g;
[0090] Viscous polymer: GMS737 30g;
[0091] The measured transdermal rate of levonorgestrel was 0.16 μg / cm 2 / h, ethinyl estradiol transdermal rate: 0.12μg / cm 2 / h.
Embodiment 3
[0093] Steroid hormones: levonorgestrel 0.3g, ethinyl estradiol 0.1g;
[0094] Viscosity modifier: PVP / VA copolymer (S-630) 0.3g;
[0095] Penetration enhancer 8g: propylene glycol 2g, isosorbide dimethyl ether 6g;
[0096] Adhesive polymer: GMS737 40g;
[0097] The measured transdermal rate of levonorgestrel was 0.20 μg / cm 2 / h, ethinyl estradiol transdermal rate: 0.12μg / cm 2 / h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com