Recombinant human endostatin temperature-sensitive gel composition for injection
A technology of vascular endothelial and composition, which is applied in the field of temperature-sensitive gel composition of recombinant human endostatin for injection, which can solve the problems of poor clinical compliance, achieve little effect on protein activity, simple and feasible process route, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
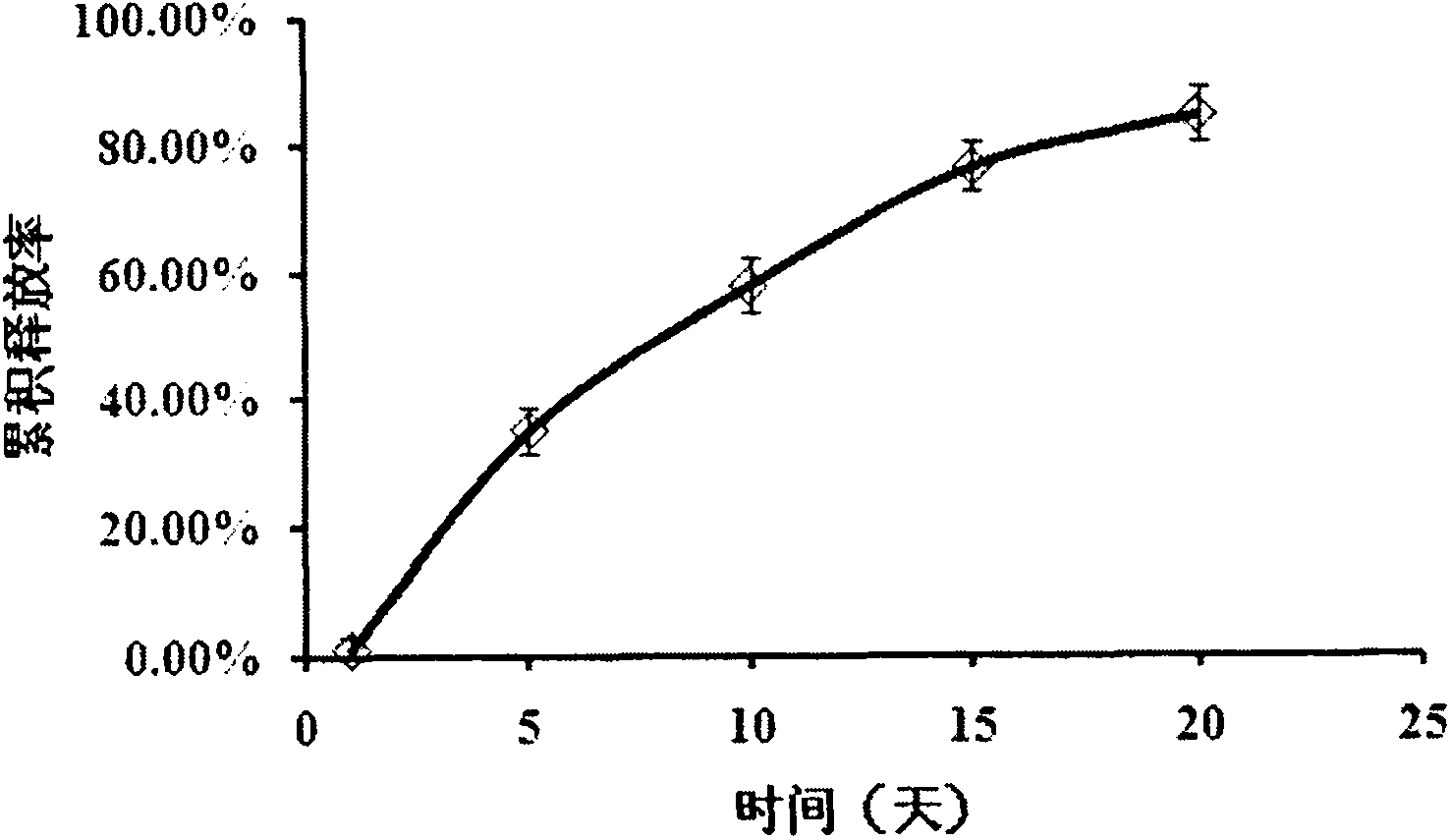
[0023] Prepare isotonic citrate buffer solution with pH 6.0, keep it refrigerated for 30min, then slowly add 10% (w / v) Poloxamer 188 and 15% (w / v) Poloxamer 407, and continue stirring to disperse Uniformly until the polymer is completely dissolved to obtain a clear solution. Take 2 mL of the above solution and place it in an ice-water bath for magnetic stirring, add 500 mg of Endostar buffer solution, stir to mix evenly, and obtain the composition. In the composition, poloxamer accounts for 16.7% of the composition, Endostar accounts for 16.7% of the composition, and the rest are biocompatibility auxiliary agents. The mixed composition was placed in a 37° C. water bath to solidify to form a sustained-release release system. The formed gel was placed in a constant temperature water bath shaker at 37°C, and 10 mL of phosphate buffer saline PBS 7.4 was added. Take 1mL of the release solution at each sampling point, and add the same volume of fresh phosphate buffer at the same t...
Embodiment 2
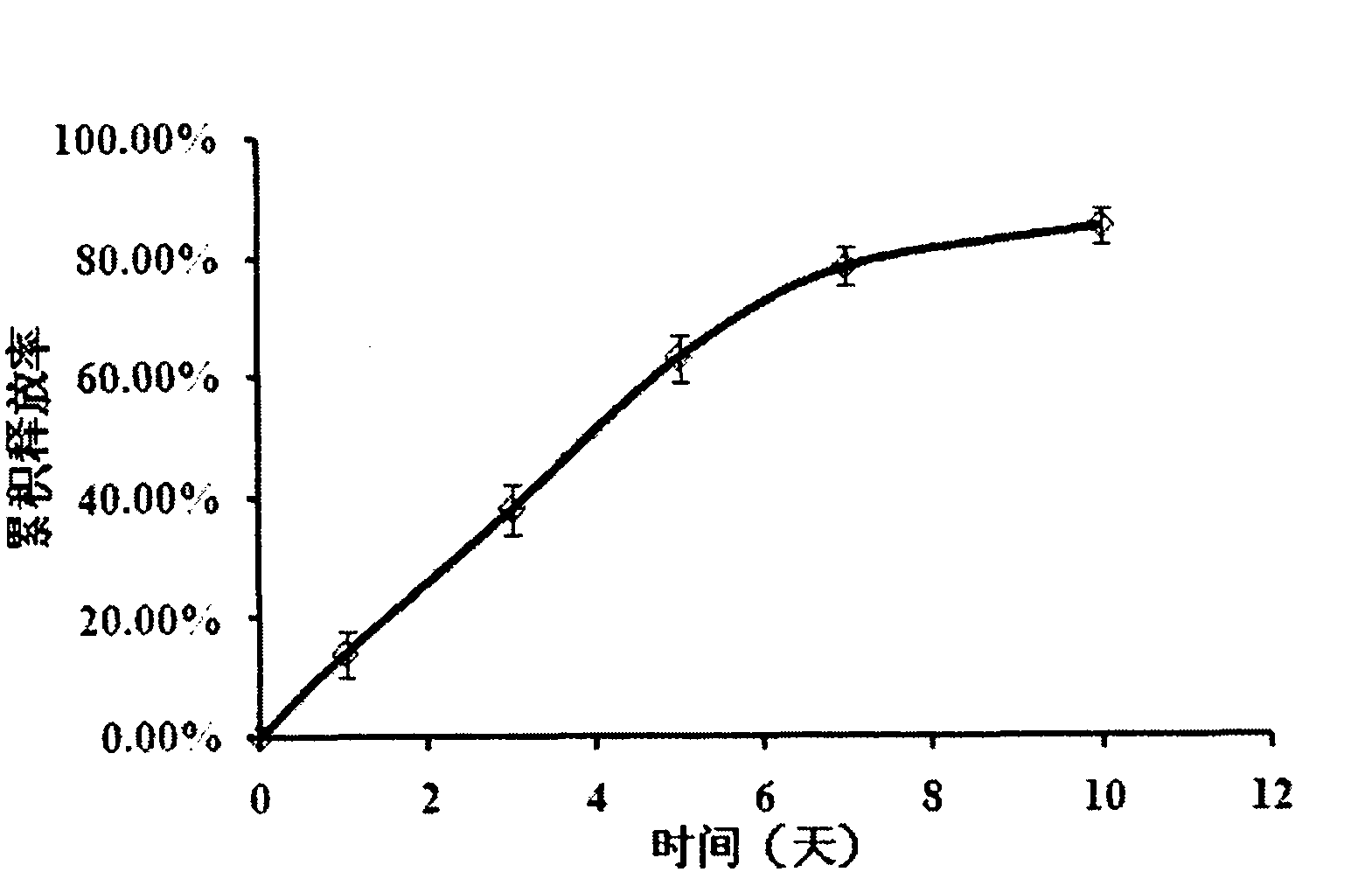
[0024] Dissolve methylcellulose in hot water above 90°C, stir to make the polymer completely wet and disperse evenly, add an appropriate amount of cold water to make the concentration 30mg / mL, stir well to dissolve, and place in a refrigerator at 4°C overnight , to obtain a clear solution. Take 1mL of the above solution and place it in an ice-water bath for magnetic stirring, add 30mg of Endostar buffer solution, 0.1mL polyethylene glycol aqueous solution (40mg / mL), 0.1mL sodium citrate aqueous solution (20mg / mL), stir to mix evenly, get the composition. In the composition, methyl cellulose accounts for 2.8% of the composition, Endostar accounts for 2.8% of the composition, and the rest are biocompatibility auxiliary agents. The mixed composition was placed in a 37° C. water bath to solidify to form a sustained-release release system. The formed gel was placed in a constant temperature water bath shaker at 37°C, and 10 mL of phosphate buffer saline PBS 7.4 was added. Take 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com