Preparation method of horse serum amyloid protein A1 and expression vector and genetic engineering bacteria thereof
An amyloid protein and genetically engineered bacteria technology, applied in genetic engineering, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problems of unstable activity, less soluble protein, and low expression, and avoid changes. Effects of renaturation steps, preservation of biological activity, and simplified purification methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
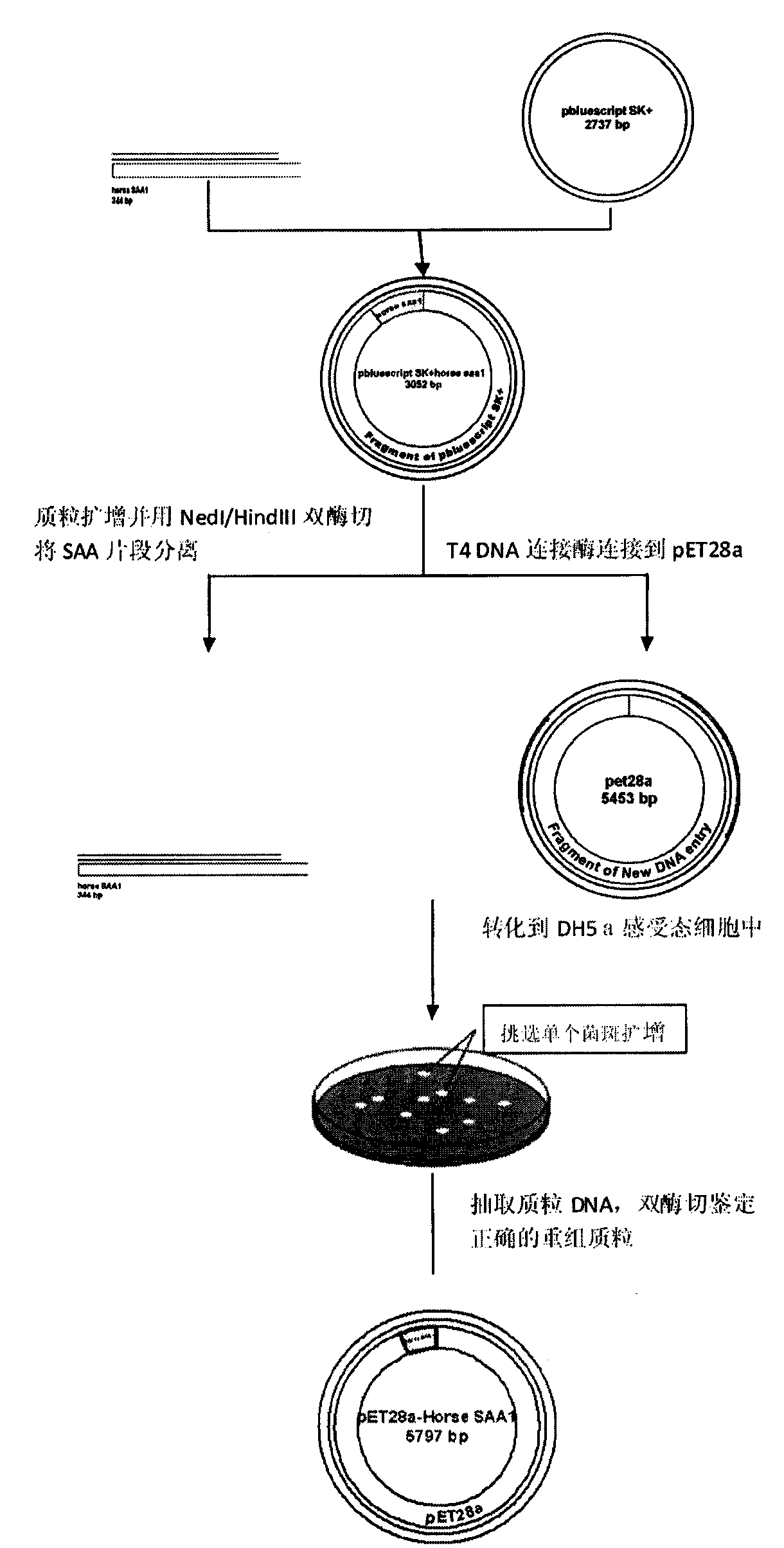
[0040] Example 1 Cloning of the gene encoding hoSAA1 and construction of the recombinant expression plasmid pET28a(+)-hoSAA1
[0041] The steps of gene cloning and plasmid construction are as follows:
[0042] (1) The hoSAA1 gene, as shown in SEQ ID NO.1, was synthesized by Invitrogen and cloned into the pBluescriptII vector;
[0043] (2) Subcloning of the hoSAA1 gene;
[0044] ①Transform the obtained pBluescriptII-SAA1 vector into DH5a host bacteria, screen and extract the plasmid containing the target DNA fragment;
[0045] ②Then undergo double enzyme digestion and separate the target DNA fragment by agarose gel electrophoresis;
[0046] ③ After purifying the target DNA fragment with a gel recovery kit, it was directly ligated to the pET-28a(+) vector;
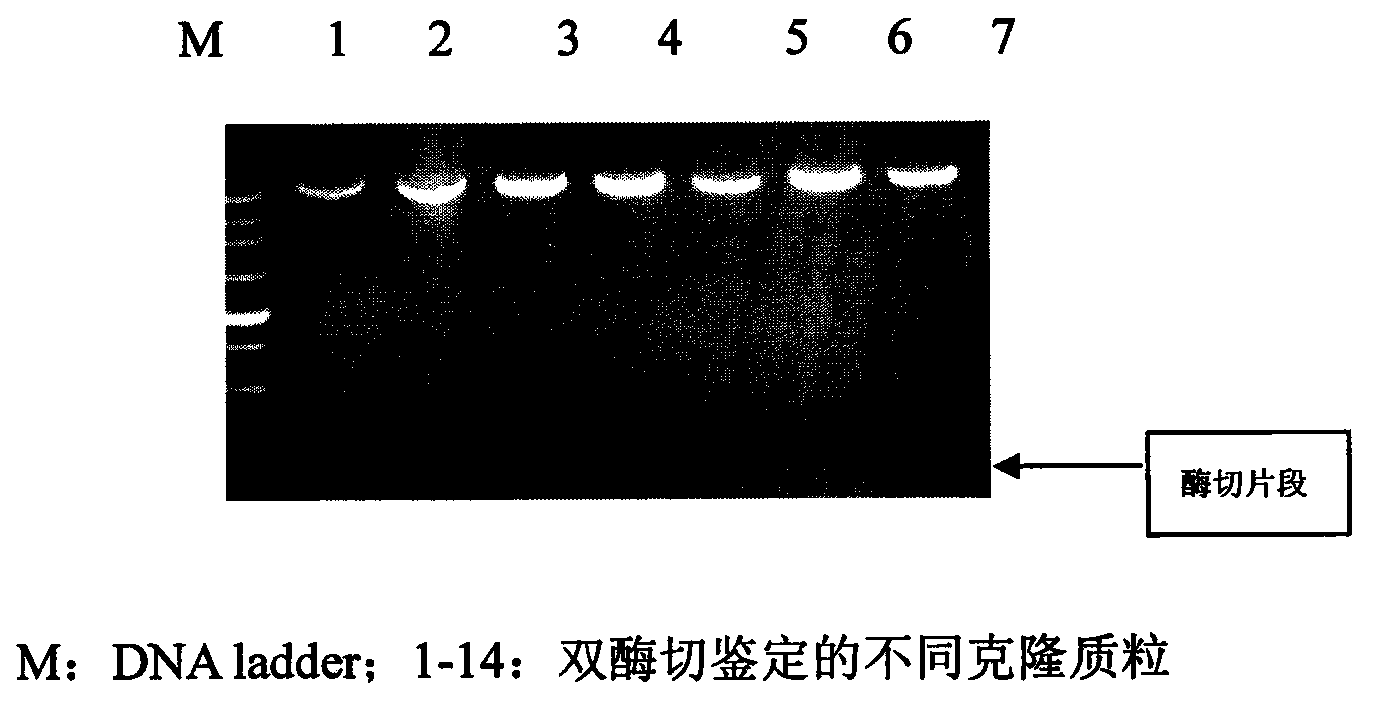
[0047] ④ Transform the ligated product into DH5a host bacteria, extract the plasmid, and identify it by double enzyme digestion with NdeI and HindIII. Among them, the size of the enzyme-digested fragment is consistent wi...
Embodiment 2
[0053] Large-scale expression and purification method of embodiment 2 gene recombinant bacteria
[0054] Expression of recombinant hoSAA1-polyhistidine fusion protein in Escherichia coli host
[0055] 1) Transform PET28a-hoSAA1 into Rosetta-gami Escherichia coli on an LB culture plate with kanamycin resistance (50ng / ml), culture at 37°C overnight;
[0056] 2) pick a single colony in 50ml LB medium containing 50μg / ml kanamycin, and culture overnight at 37°C and 250 rpm;
[0057] 3) 2% was inoculated into 1L LB medium (including 50ug / ml kanamycin), and the bacteria were cultivated to A600 at 37°C and 250 rpm, with an O.D value of 0.5;
[0058] 4) Add IPTG to a final concentration of 1 mM and induce culture at 37°C and 300 rpm for 3 hours;
[0059] 5) Centrifuge at 4000 rpm for 20 minutes at high speed, collect the precipitate and store it at -80°C.
[0060] Reagent for purifying recombinant hoSAA1-polyhistidine fusion protein
Embodiment 3
[0076] Embodiment 3 Western blotting proves to recombinant protein
[0077] 1) 15% acrylamide glue, 90V, 3 hours;
[0078] 2) Electrotransfer the protein fragments on the acrylamide gel to PVDF membrane through Tris-glycine buffer solution, the conditions are 1X Tris-glycine buffer solution, 20% methanol, 70V voltage for 3 hours at room temperature;
[0079] 3) Take out the PVDF membrane after electrotransfer and wash it with 1X PBST for 30 minutes;
[0080] 4) block with PBST containing 5% skimmed milk powder for 45 minutes;
[0081] 5) Rinse the PVDF membrane with 1X PBST 3 times, 10 minutes / time;
[0082] 6) Add 8 mL of PBST containing 1:2000 diluted anti-His antibody, and react at room temperature for 1 hour;
[0083] 7) Wash the PVDF membrane three times with 1X PBST, 15 minutes / time;
[0084] 8) Add anti-mouse antibody containing 1:1000 dilution and react at room temperature for 40 minutes;
[0085] 9) Wash the PVDF membrane three times with 1X PBST, 15 minutes / time...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com