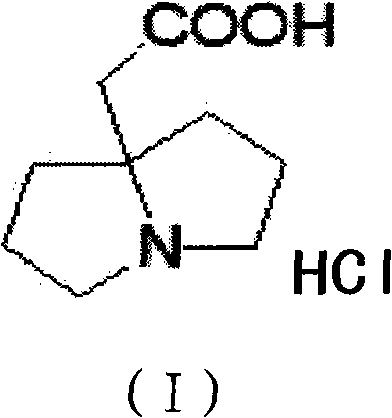
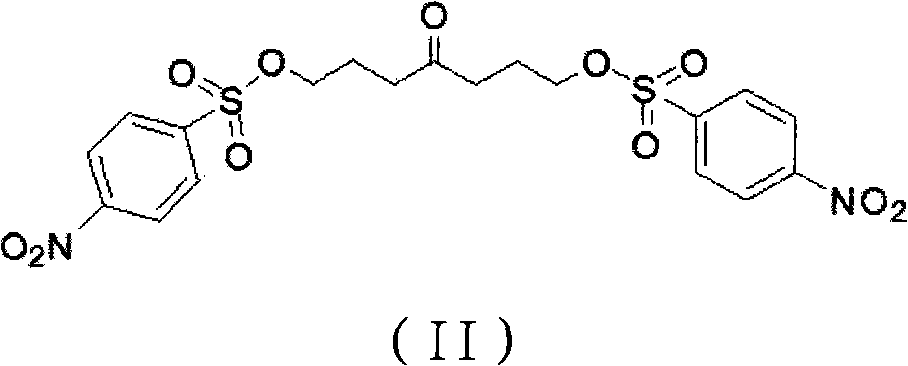
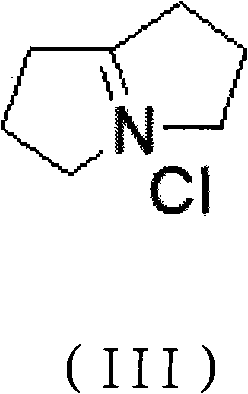
Preparation method of pyrrolizidine-9-acetic acid hydrochloride
A technology of double-condensed pyrrolidine chlorate and double-condensed pyrrolidine, which is applied in the field of preparation of double-condensed pyrrolidine-9-acetic acid hydrochloride, can solve the problem that the preparation method and extraction amount of double-condensed pyrrolidine are not given. Less, complex process and other problems, to achieve the effect of simplifying the preparation process, optimizing the reaction temperature, and improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] Step 1) preparation of double-condensed pyrrolidine chlorate;
[0048] Step 2) preparation of double-condensed pyrrolidine-9-acetocyanide;
[0049] Step 3) Preparation of double-condensed pyrrolidine-9-acetic acid hydrochloride.
Embodiment 1
[0051] (1) Preparation of double-condensed pyrrolidinium chlorate
[0052] According to the method disclosed in the former Soviet Union Patent SU1351919A, 1,7-di-p-nitrobenzenesulfonate-4-heptanone was prepared.
[0053] Connect a 2000ml four-necked explosion-proof reaction flask with a low-temperature cooling circulation device and a thermometer. Add 675ml of concentrated ammonia water and 240g of ammonium chloride to the reaction flask, use a low-temperature cooling device to cool the temperature of the reaction solution to -10°C, then add 536g of 1,7-di-p-nitrobenzenesulfonate-4-heptanone, and pass Add ammonia gas, increase the pressure in the container to 6-8MPa, control the reaction temperature at 5-10°C, and react for 15 hours.
[0054] After the reaction, the reaction solution was spin-dried, and the solid was washed with 500ml of dichloromethane (DCM), filtered with suction, and the mother liquor was spin-dried to obtain 125g of a light yellow crude product, which was...
Embodiment 2-4
[0060] Embodiment 2-4 is to illustrate the yield of double-condensed pyrrolidinium chlorate during different charging ratios, and other implementation steps are referring to embodiment 1.
[0061] According to the steps described in Example 1, double-condensed pyrrolidinium chlorate is prepared, wherein 1,7-di-p-nitrobenzenesulfonate-4-heptanone and the feeding amount of concentrated ammonia water and the corresponding product amount and yield respectively As shown in Table 1.
[0062] Product amount and yield table of double condensed pyrrolidinium chlorate of table 1
[0063] Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com