Method for preparing cefuroxime sodium
A technology of cefuroxime sodium and cefuroxime acid, which is applied in the field of drug synthesis, can solve problems such as visible foreign matter and difficulty in aseptic control, prolonged post-processing steps, and increased product exposure opportunities, so as to reduce material powder exposure and human contact opportunity, short dissolution time, and easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of Cefuroxime Sodium
[0021] Add 200ml of acetone and 30ml of purified water to a 500ml three-necked bottle, put in 40g of cefuroxime acid, stir at a temperature of 25°C until it is completely dissolved, add 4g of activated carbon, filter with suction, and add a completely dissolved solution to the filtrate at a temperature of 25~-30°C. The mixed solution of sodium isooctanoate, sodium acetate and sodium lactate was added and stirred for 15 minutes, 600ml of acetone was added dropwise to crystallize, slowly stirred for 30 minutes to grow the crystals, suction filtered, washed with acetone and dried for 2 hours to obtain the finished product of cefuroxime sodium. The content is 94.8%, and the maximum impurity is 0.2%.
Embodiment 2
[0022] Example 2: Detection of Cefuroxime Sodium Product Properties
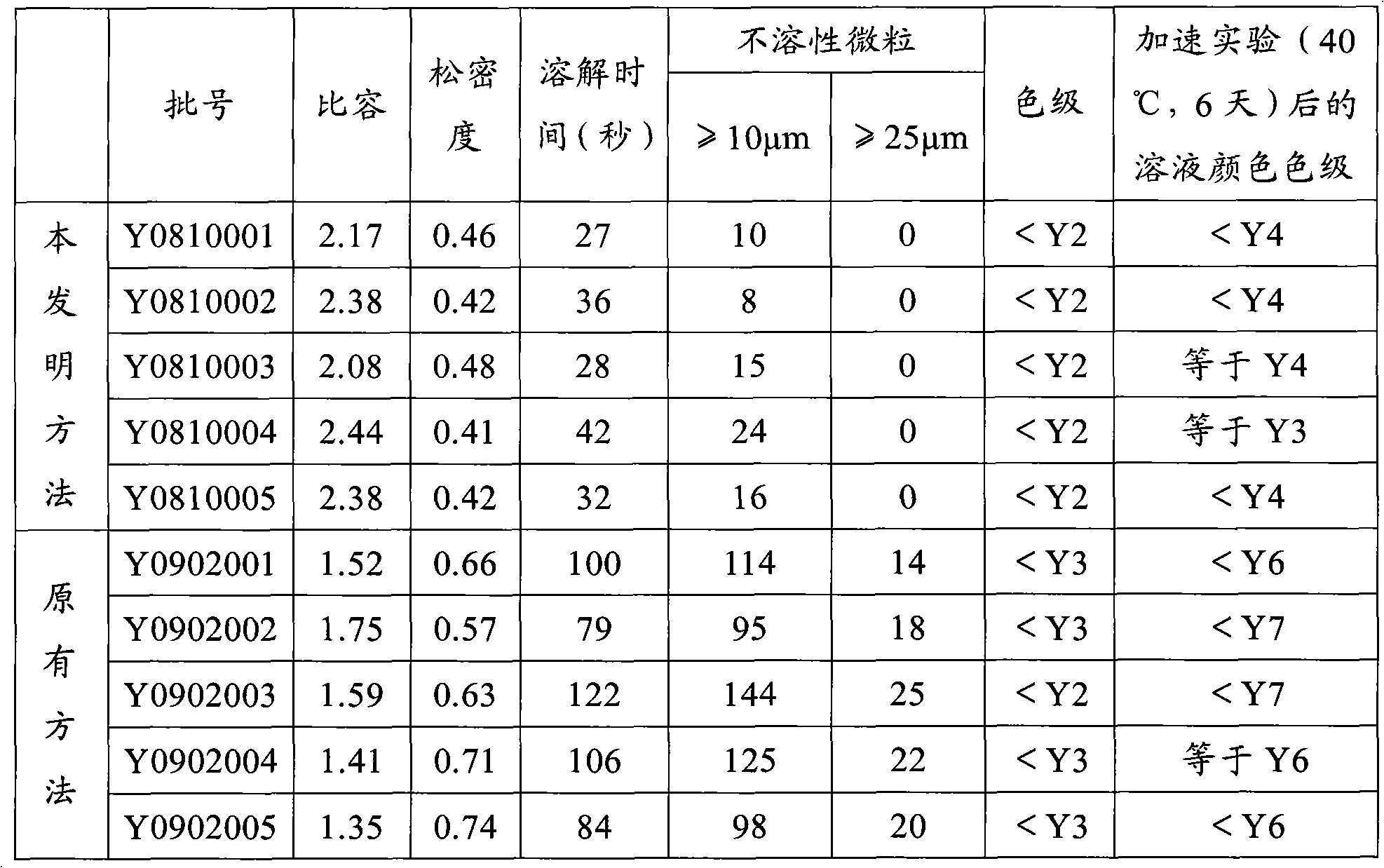
[0023] Five batches of cefuroxime sodium products were prepared according to the preparation method in Example 1, and the batch numbers were Y0810001, Y0810002, Y0810003, Y0810004 and Y0810005, and the following items were tested for these five batches of cefuroxime sodium products. The results are shown in Table 1
[0024] Table 1 Test results of cefuroxime sodium product properties
[0025]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com