Growth factor-loaded collagen group composite material as well as preparation method and application thereof
A growth factor and composite material technology, applied in medical science, prosthesis, etc., can solve the problems of uncontrolled growth factor release direction, loss of growth factor activity, waste of growth factor, etc., to achieve good biocompatibility, preparation process Mild conditions and structurally stable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of loose and dense collagen membranes
[0026] 1. Preparation of bovine tendon type Ⅰ collagen swelling solution
[0027] The fresh beef tendons purchased in the market were fully washed, the peritendon tissue, tendon sheath and fascia were removed, and after washing, they were frozen in a freezer. Take out the frozen beef tendon and cut it into slices with a thickness of about 1 mm, add ficin solution (concentration 0.05-0.25%) for treatment, stir and put it in an incubator for enzymolysis for 24 hours, then add excess H 2 o 2 Terminate the reaction, wash with distilled water 3-4 times, add 0.3% malonic acid solution to swell for 24 hours after decanting. The swollen tendons were fully stirred for 6 hours, the viscosity was adjusted with 0.3% malonic acid solution, and filtered with 80-120 mesh stainless steel mesh under positive pressure to remove impurities and unswollen substances, and then packaged.
[0028] 2. Preparation of air-dried col...
Embodiment 2
[0038] Embodiment 2: Preparation of chitosan-heparin-bFGF nanoparticles by ion cross-linking
[0039] 1. Chitosan (molecular weight 10000, degree of deacetylation 85%) was dissolved in 1% acetic acid solution, filtered and dialyzed for 4 days, freeze-dried to obtain purified chitosan.
[0040] 2. Weigh 0.2g chitosan and dissolve in 5ml 1% acetic acid, 0.1mol L -1 Adjust the pH to 6.0 with NaOH, set the volume to 100ml, and obtain a concentration of 2mg·ml -1 Chitosan solution is filtered to remove impurities therein.
[0041] 3. At 1mg·ml -1 Add the expected amount of bFGF to the heparin (molecular weight: 12,000) solution and mix overnight (8-12 hours). At 4°C, with stirring at 700rpm, 2ml of 2mg·ml -1 Chitosan solution drop by drop into 5ml 1mg·ml -1 In the composite bFGF heparin, the dropping speed is 20 drops / min, and the chitosan-heparin nanoparticles loaded with bFGF are obtained.
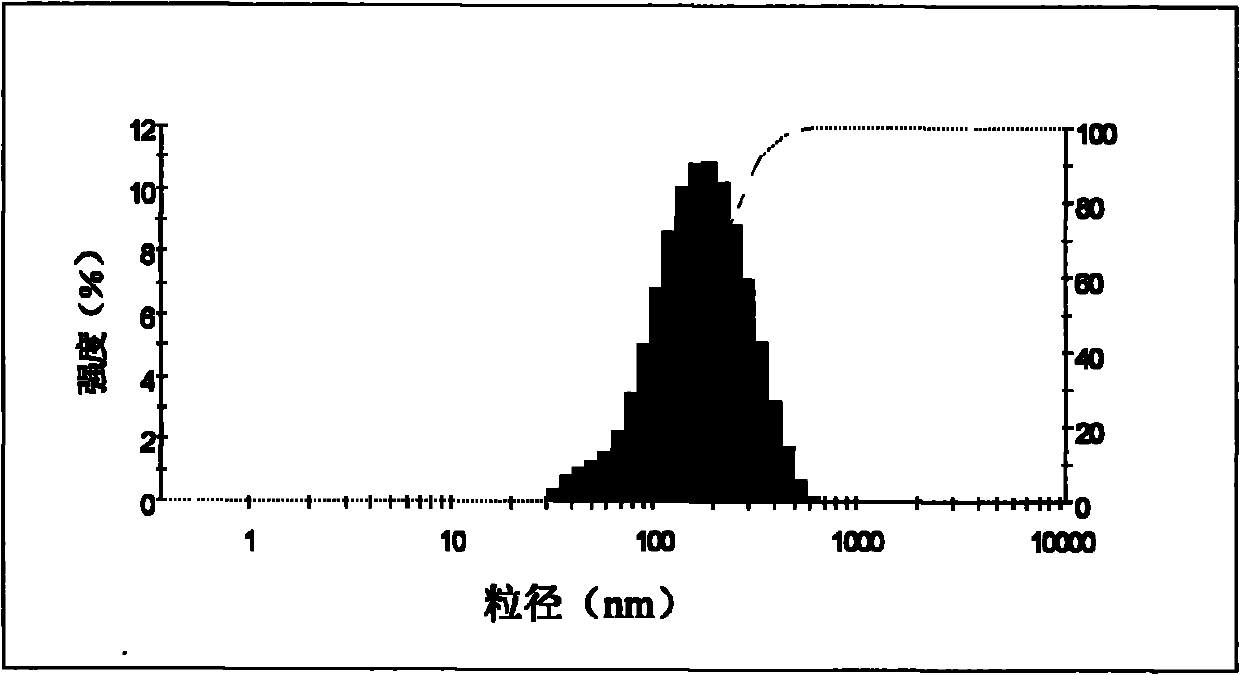
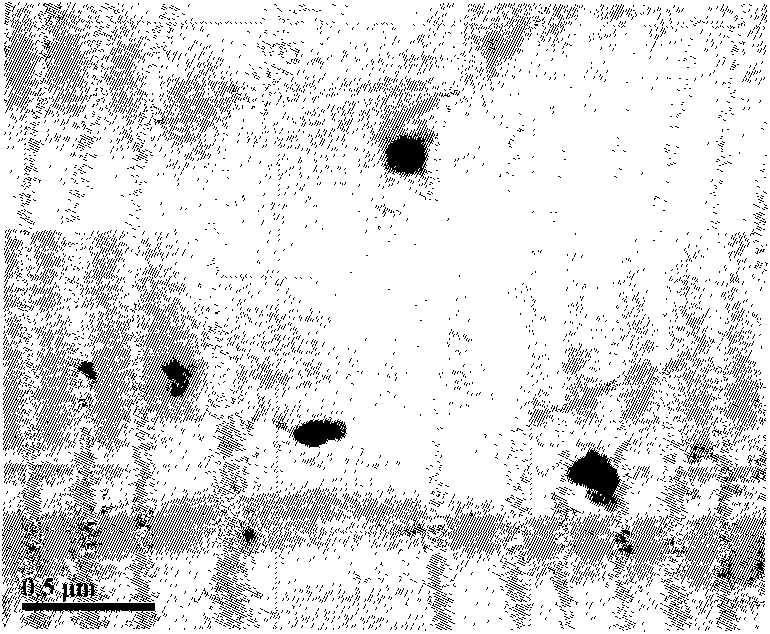
[0042] The prepared nanoparticles are relatively uniform in size observed under tr...
Embodiment 3
[0043] Example 3: Preparation of bFGF-loaded collagen-based composite material
[0044] Wet the cross-linked loose layer collagen membrane prepared in the above example with double distilled water and spread it flat, and slowly drop the Ch-He nanoparticles loaded with bFGF on the surface to cover the entire surface. Air-dry at 4°C until there is no mobile phase liquid on the surface of CFF, then wet the cross-linked dense layer collagen film and spread it on the surface of the cross-linked loose layer collagen film with a small amount of liquid remaining, and air-dry thoroughly at 4°C to obtain bFGF-loaded collagen Matrix composite material, wherein the amount of bFGF is 100ng / cm -2 .
[0045] Spray gold on the cross-section of the double-layer material, and it can be seen by field emission scanning electron microscopy ( image 3 ): The composite material is composed of two layers. The dense layer structure is dense and smooth, and the loose layer is loose and porous. This s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com