Method for synthesizing octadecyl methyl dihydroxyethyl ammonium bromide by using microwave
A technology of octadecyl methyl dihydroxyethyl ammonium bromide, microwave synthesis, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve problems such as no reports, and achieve energy consumption The effect of less, higher conversion rate and higher reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
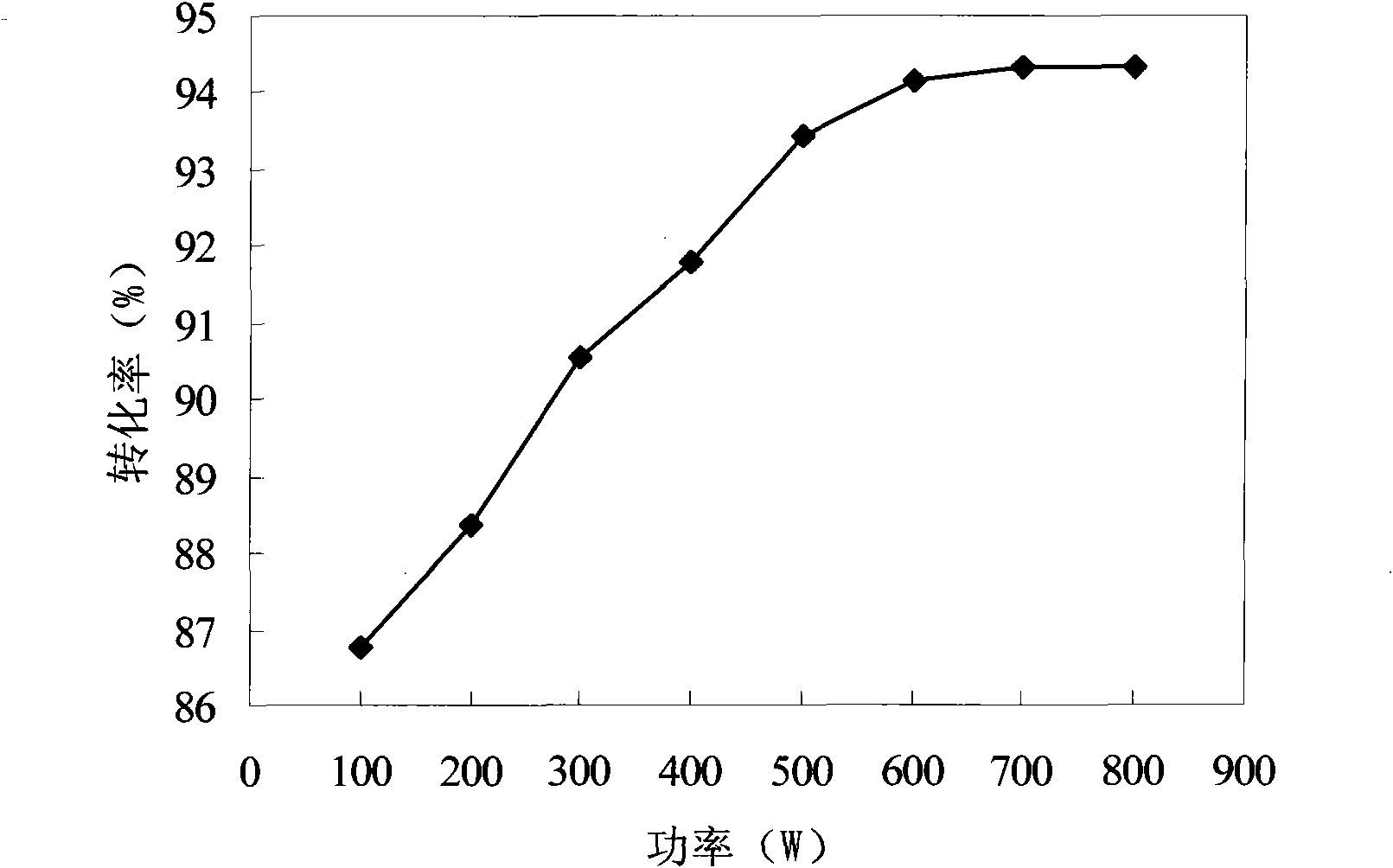
[0047] Add N-methyldiethanolamine (0.02mol) and bromooctadecane (0.02mol) in a molar ratio of 1:1 into a flat-bottomed flask, and measure 10ml of ethanol, n-propanol, n-butanol, and n-pentanol respectively and n-hexanol were dissolved and mixed evenly, and the microwave reflux synthesis was carried out under the condition that the microwave power was 600W and the reaction time was 25min.
[0048] The conversion rates of the above reactions were evaluated, and the results are shown in Table 1.
[0049] The influence of table 1 solvent on conversion rate
[0050]
[0051]It can be seen from the results that in the above 5 reactions, the reaction solution gradually turned from colorless to yellow, and the color gradually deepened and the viscosity increased. The color of the product can directly reflect the conversion rate of the reactant. As can be seen from the above table, the conversion rate is the highest when n-hexanol is used as solvent. This may be because the polar...
Embodiment 2
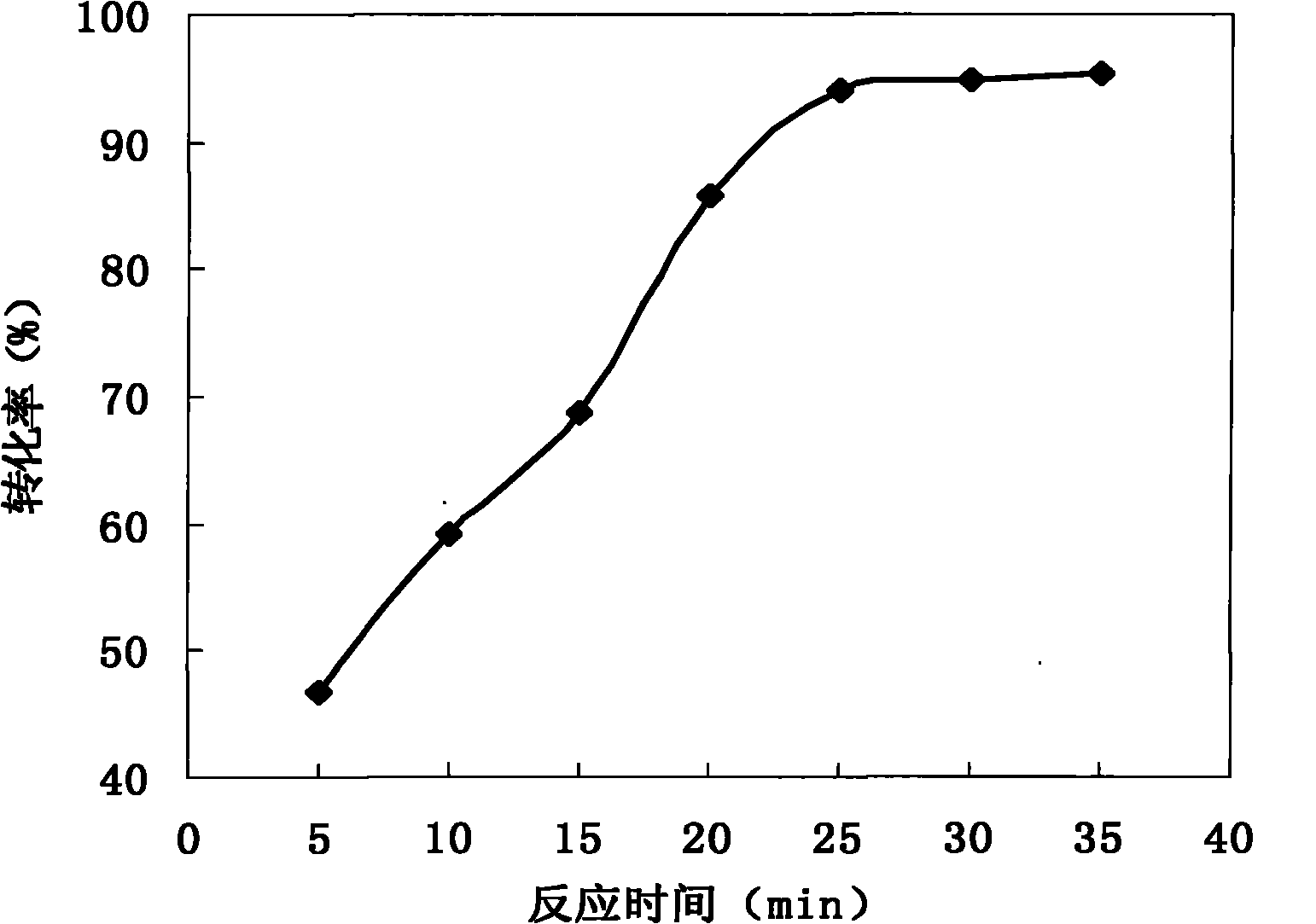
[0053] Add N-methyldiethanolamine (0.02mol) and bromooctadecane (0.02mol) with a molar ratio of 1:1 into a flat-bottomed flask, measure 10ml of n-hexanol, mix and dissolve, and react separately under a microwave power of 600W 5, 10, 15, 20, 25 minutes. see results figure 1 shown.
[0054] As can be seen from the results, the reaction time will have a higher conversion rate above 20min, this is due to the prolongation of time, the probability of collision between molecules increases greatly, so that the reaction tends towards completeness, when the reaction time is 25min, the conversion rate is 93.87%. Although the reaction time was further extended later, the increase in the conversion rate was not large. This may be because the concentration of the reactants decreased, which made the gradient of the reaction decrease. At the same time, based on the consideration of production energy consumption, 15-25min was selected as the microwave irradiation time. the optimization rang...
Embodiment 3
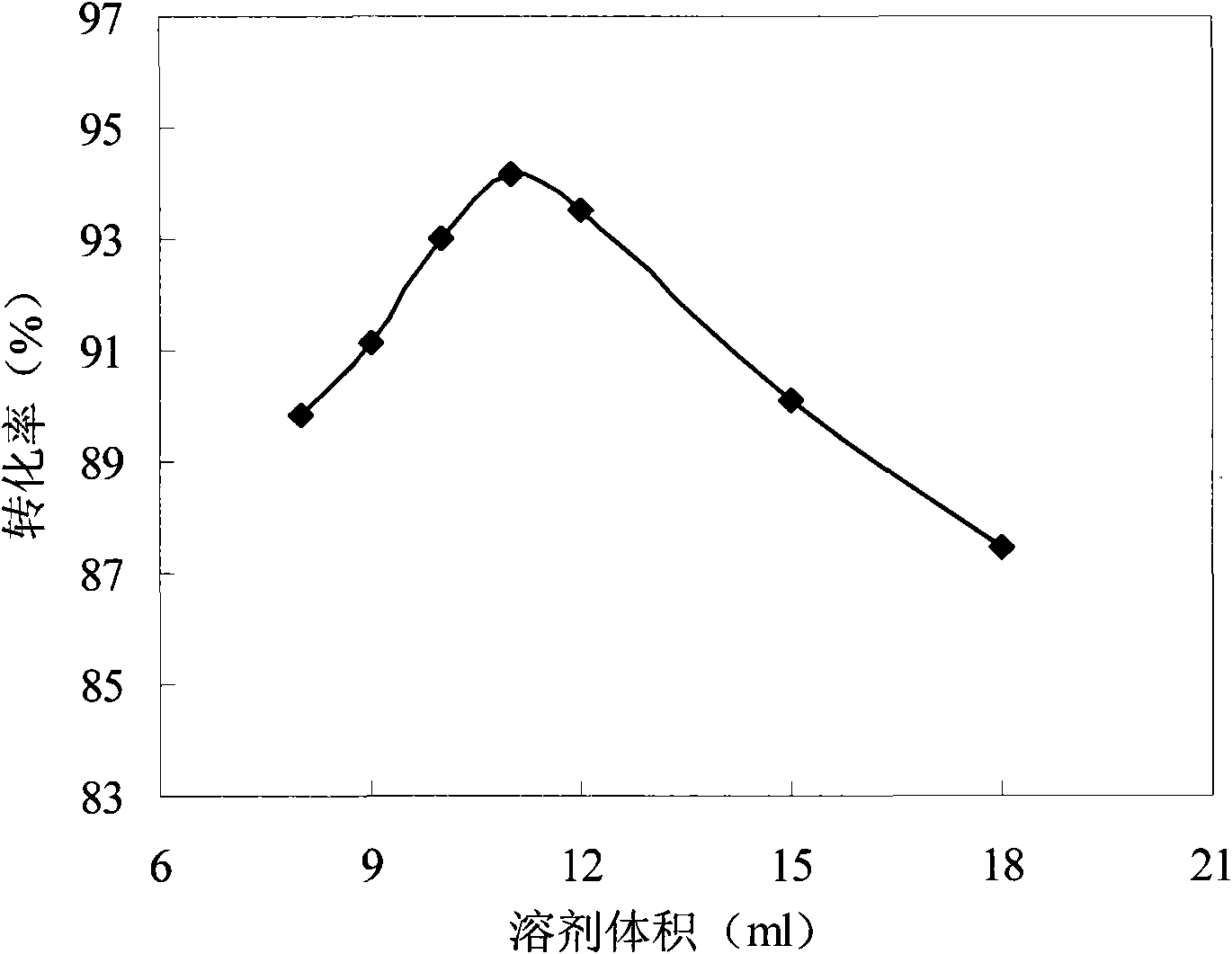
[0056] Add N-methyldiethanolamine (0.02mol) and bromooctadecane (0.02mol) in a molar ratio of 1:1 into a flat-bottomed flask, and measure 8, 9, 10, 11, 12, 15, 18ml of n-hexanol, under the microwave power of 600W, react for 25min. The result is as figure 2 shown.
[0057] It can be seen from the results that the conversion rate increases with the increase of the solvent volume, and the conversion rate is the largest when the solvent volume is 11 mL, but the conversion rate decreases when the solvent volume continues to increase. This is because the volume of the solvent is too small and the viscosity is too high, which is not conducive to the reaction. Increasing the volume of the solvent and the uniform mixing of the reactants increases the probability of collision between molecules, which is conducive to the reaction. However, as the volume of the solvent continues to increase, the solvent will absorb more energy from the reaction system, reducing the energy obtained by t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com