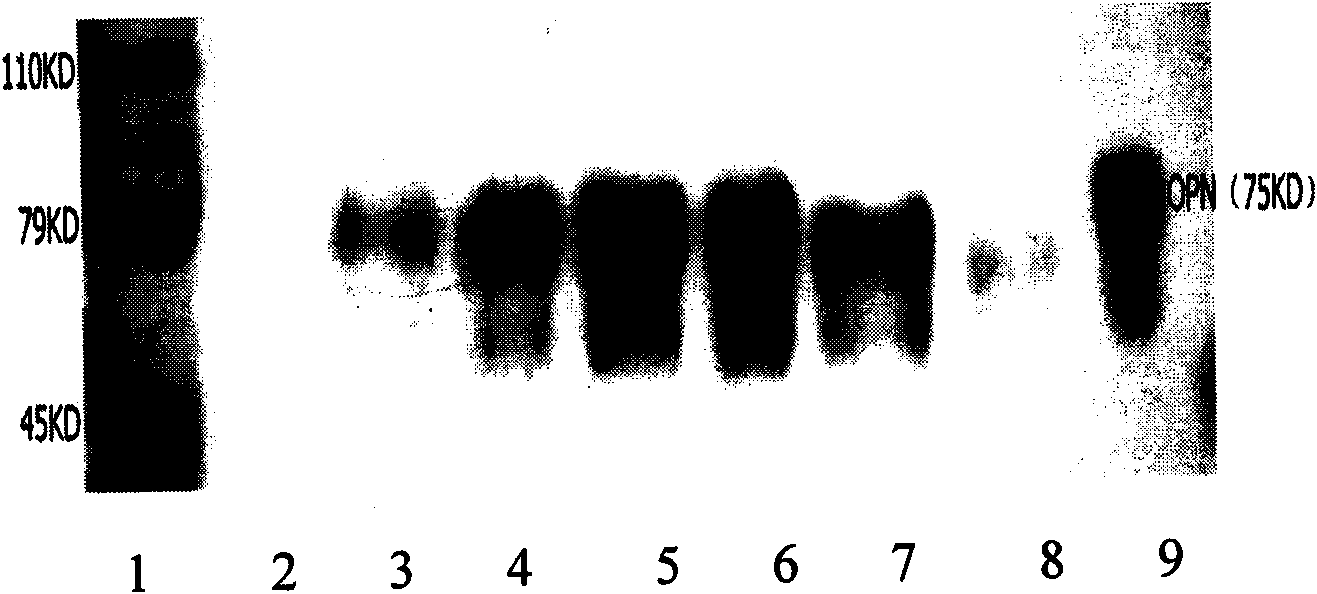Anti-osteopontin OPN monoclonal antibody and application thereof
A monoclonal antibody and osteopontin technology, which is applied in the direction of antibodies, anti-animal/human immunoglobulins, anti-tumor drugs, etc., can solve the problem that there is no good standard for OPN detection, affecting OPN clinical detection, and antibody missed detection And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
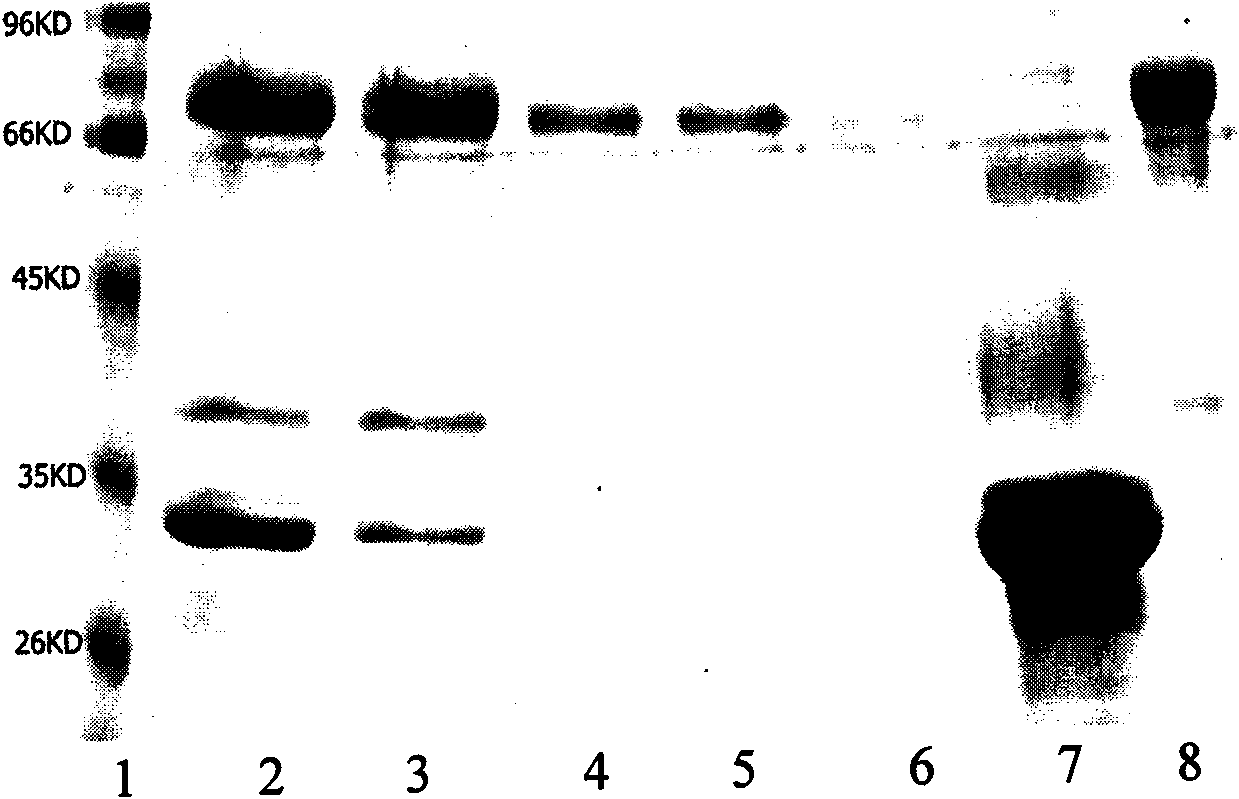
Image
Examples
Embodiment 1
[0151] Example 1: Purification of native OPN from human milk
[0152] The present inventors used an optimized two-step method to extract natural OPN from milk. The separation and purification method of the present invention provides a new method for obtaining natural OPN. After long-term exploration and experiments, the inventors optimized a two-step chromatography method to obtain OPN with a purity of up to 95% from milk, which not only shortened the separation steps and purification time, but also reduced the loss of OPN and activity loss. The specific steps are:
[0153] 1. Milk processing: Centrifuge 200ml of milk (from lactating women) at 1000rpm / 10min, remove the upper layer of milk fat, and reserve the whey for the next step of purification.
[0154] 2. The ion exchange method enriches OPN in milk and removes most of the foreign proteins.
[0155] Materials: whey (200ml); DEAE sepharose (GE, 20ml); GE company Akta Purify purifier.
[0156] Process: After equilibrati...
Embodiment 2
[0172] Example 2: Animal immunization
[0173]Select healthy BALB / c mice homologous to the myeloma cells used, and the age of the mice is 8-12 weeks, male or female. The antigen is the natural purified OPN protein prepared in Example 1. Antigen stock solution concentration: 1 mg / ml; Balb / c mouse immunization dose: 100 μg OPN per mouse. The injection method is multi-point intramuscular injection. Dilute with PBS or normal saline before use. Immunization schedule: three immunizations in weeks 0, 3 and 6. Three days before the fusion, take 100μg and dilute it with PBS to 0.5ml for intraperitoneal injection for memory stimulation. Three days after the last immunization, splenocytes were isolated for fusion. Immunization results are shown in Table 1 below.
[0174] Table 1: Antibody titers in mouse sera after the third immunization with OPN
[0175]
Embodiment 3
[0176] Example 3: Construction of hybridoma cell lines and preparation of monoclonal antibodies
[0177] 1. Culture of myeloma cell lines
[0178] The most important point in selecting tumor cells is homology with the B cells to be fused. If spleen cells are to be fused, various myeloma cell lines can be used, and we use SP2 / 0 cell line. The growth and fusion efficiency of the cell line is average. In addition, the cell line itself does not secrete any immunoglobulin heavy chain or light chain. The highest growth scale for cells is 9*10 5 / ml, the doubling time is usually 10-15h. For fusion cells, select cells in the logarithmic growth phase, cell shape and activity (the activity is greater than 95%). The myeloma cell lines were first adapted to culture medium containing 8-azaguanine before fusion, and the day before cell fusion, fresh medium was used for 2*10 5 / ml, the next day is generally logarithmic growth phase cells.
[0179] 2. Culture of feeder cells
[0180] U...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com