Uncaria sustained-release capsule preparation and preparation method thereof
A technology of sustained-release capsules and preparations, applied in the field of Uncaria sustained-release capsule preparations and its preparation, which can solve the problems of lack of sustained-release effect, poor curative effect, and inability to control blood pressure smoothly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
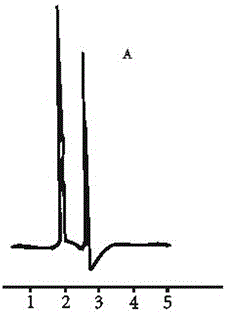
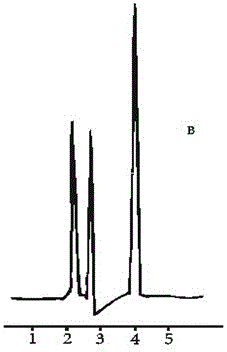
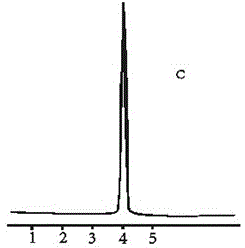
Image
Examples
Embodiment 1
[0065]Embodiment 3: first take by weighing 85g starch, 50g microcrystalline cellulose, 88g dextrin, sieve, mix uniformly, with 10g ethyl cellulose as wetting agent, make blank ball core in coating granulator, Dry and set aside. Then weigh 6.5g of rhynchophylline, 43g of starch, and 1.0g of sodium lauryl sulfate to pulverize and sieve respectively, place the blank ball core in the coating pan, add the mixture of rhynchophylline, starch and sodium lauryl sulfate The mixture is sprayed with ethyl cellulose, then the powder mixture is added, the ethyl cellulose is sprayed, and the operation is repeated until all the powders are added. After dosing, take it out and place it on a tray to dry. Finally, 5 g of hydroxypropyl methylcellulose was weighed and added to 200 ml of 70% ethanol, and left overnight. Add 15 g of diethyl phthalate and 1.0 g of titanium dioxide, and stir evenly to make a coating liquid. Put the above-mentioned vegetarian pills in the coating pot, then spray the...
Embodiment 2
[0066] Embodiment 4: First take by weighing 100g starch, 50g sodium carboxymethylcellulose, 77g dextrin, sieve, mix evenly, use polyvinylpyrrolidone and ethylcellulose mixture solution as wetting agent, in coating granulator Make a blank ball core, dry it, and set it aside. Then weigh 17g of total alkaloids of Uncaria, 52g of starch, and 10g of Tween, respectively pulverize and sieve, put the blank ball core in the coating pan, add the mixture of total alkaloids of Uncaria, starch and Tween, and spray into hydroxypropyl formazan. Base cellulose solution, then add the powder mixture, spray into the solution, repeat the operation until all the powder is added. After dosing, take it out and place it on a tray to dry. Finally, 12 g of hydroxypropyl methylcellulose was weighed and added to 250 ml of ethanol, and left overnight. Add 38 g of diethyl phthalate and 1.0 g of titanium dioxide, and stir evenly to make a coating liquid. Put the above-mentioned vegetarian pills in the co...
Embodiment 3
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com