Method for recovering copper, zinc and iron from sulphide ore calcine pickle liquor containing gold and silver
A technology of sulfide ore and calcine acid, which is applied to recover non-ferrous metals in Ag sulfide ore calcine acid leaching solution, and can solve the problems of complex process, pollution of working environment of organic extractant, pollution of environment by heavy metal ions, etc. from the field containing Au. Achieve the effect of simple process, less environmental pollution and low recovery cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
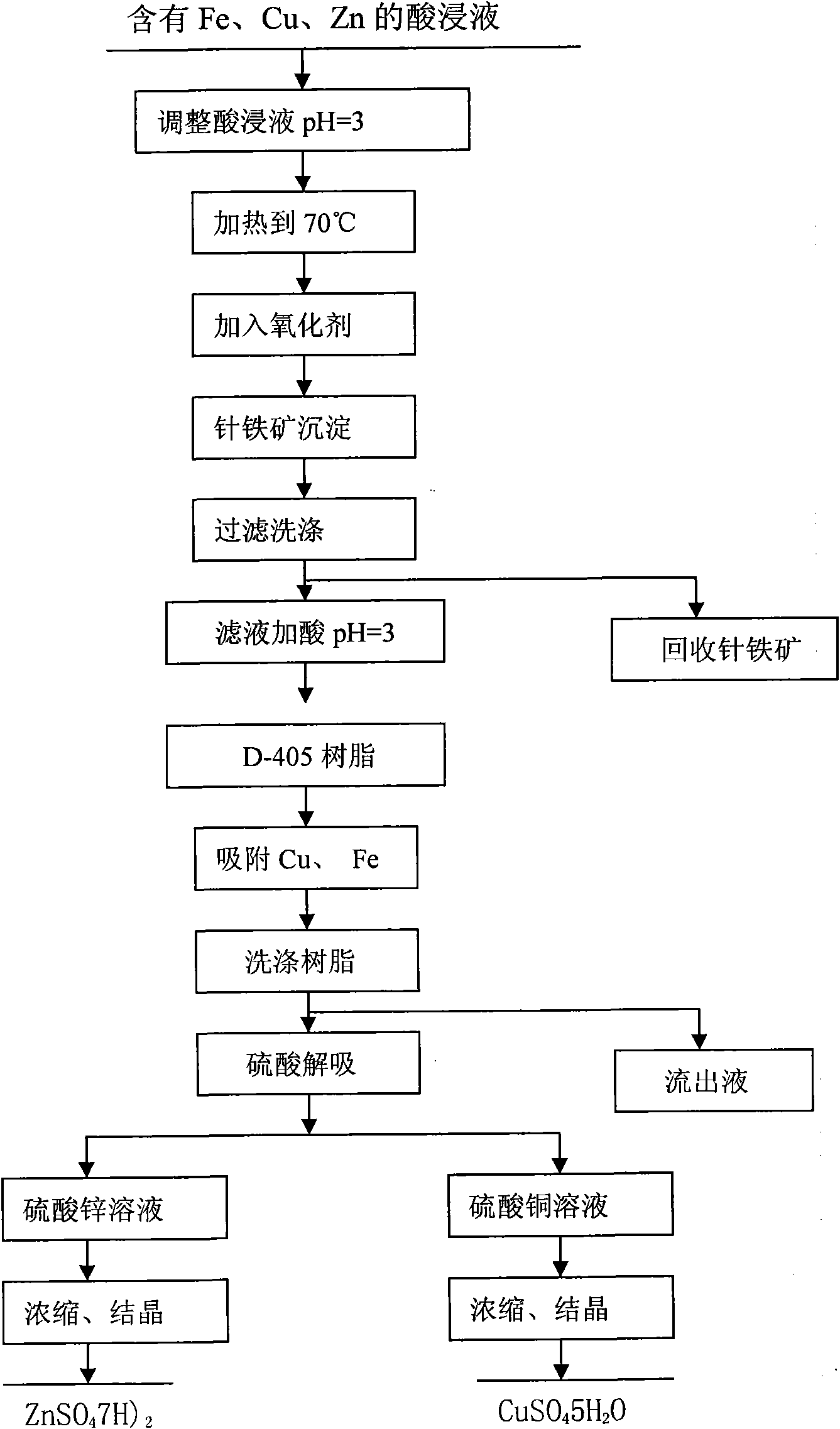
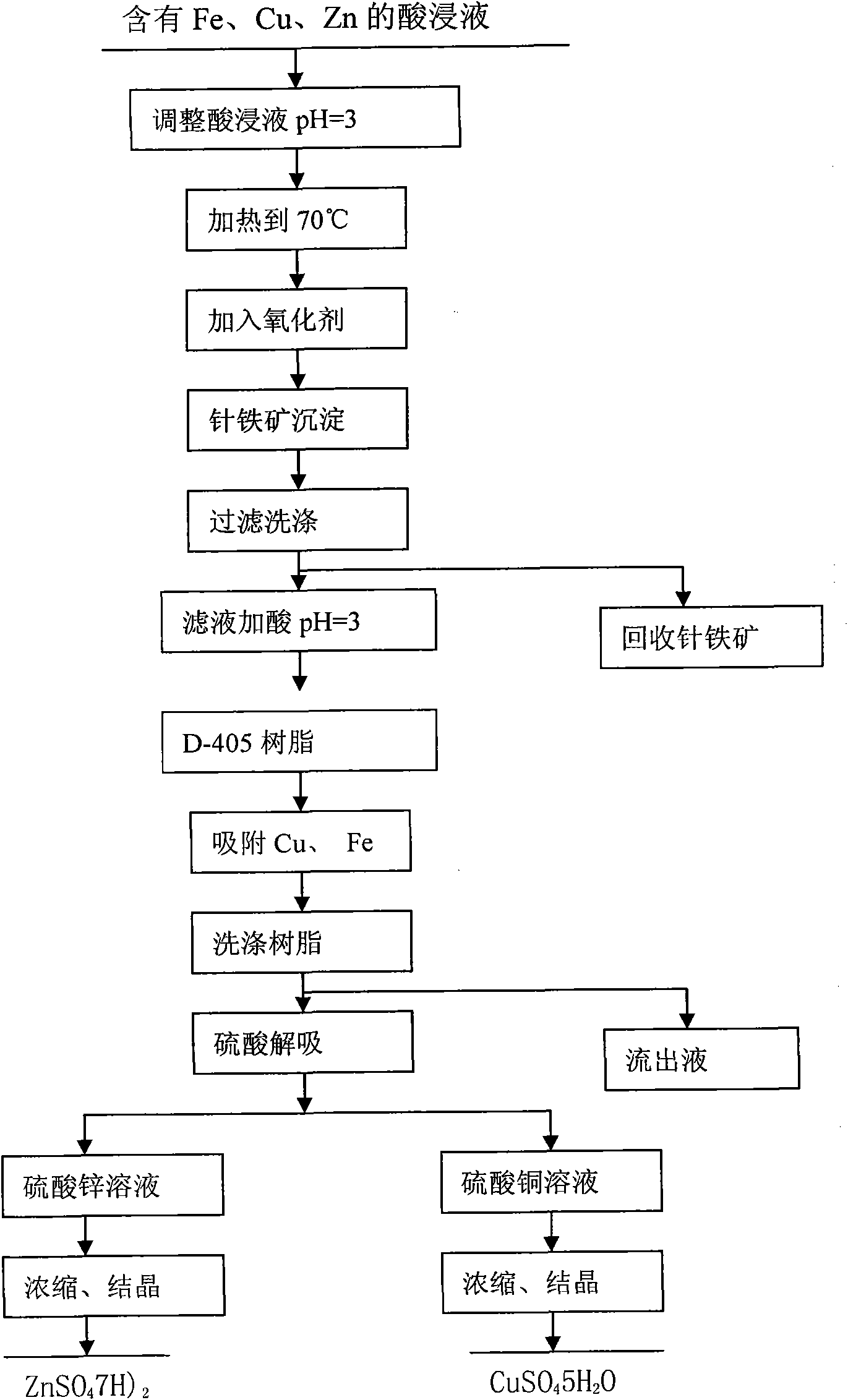
[0035] a. Adjust the acid dipping solution containing Fe, Cu and Zn to pH = 3, then heat it to 70°C, add air under the condition of stirring, and the air speed is 0.025m 3 / min, stirring the reaction for 6h, so that the Fe in the solution +3 Ions completely form goethite precipitates;
[0036] b. Recycling goethite through filtration and washing;
[0037] c, adding sulfuric acid to the copper-containing and zinc-containing filtrate after the goethite is adjusted to pH=3;
[0038] d. Through the D-405 resin column, carry out Cu and Zn adsorption for 1 hour;
[0039] e. Wash the D-405 resin after absorbing Cu and Zn with deionized water to remove impurities, and desorb Zn with 0.2mol / L sulfuric acid solution;
[0040] f. Desorb Cu with 1.0mol / L sulfuric acid solution on the D-405 resin after desorbing Zn;
[0041] g. Adjust the desorbed zinc sulfate solution to pH=5.0, heat to evaporate→concentrate→crystallize, heat and evaporate to a density of 1.53g / mL, stop heating, and c...
Embodiment 2
[0044] a. Adjust the acid dipping solution containing Fe, Cu and Zn to pH = 3, then heat to 70°C, and add H under stirring 2 o 2 , H 2 o 2 The amount added is Fe in the solution 3+ Stop adding when the yellow color fades; stir and react for 5.5 hours, so that the Fe in the solution completely forms goethite precipitation;
[0045] b. Recycling goethite through filtration and washing;
[0046] c, adjusting the filtrate containing Cu and Zn after recovering the goethite to pH=3;
[0047] d. Through the D-405 resin column, carry out Cu and Zn adsorption for 1.5 hours;
[0048]e. Wash the D-405 resin after absorbing Cu and Zn with deionized water to remove impurities, and desorb Zn with 0.2mol / L sulfuric acid solution;
[0049] f. The D-405 resin after desorbing zinc is then desorbed Cu with 1.0mol / L sulfuric acid solution;
[0050] g. Adjust the desorbed zinc sulfate solution to pH=5.0, heat to evaporate→concentrate→crystallize, heat and evaporate to a density of 1.55g / mL,...
Embodiment 3
[0053] a. Adjust the acid immersion solution containing Fe, Cu and Zn to pH = 3, then heat it to 70°C, add air under the condition of stirring, and the speed of the air is 0.025m 3 / min, stirring the reaction for 5h, so that the Fe in the solution +3 Ions completely form goethite precipitates;
[0054] b. Recycling goethite through filtration and washing;
[0055] c, adding sulfuric acid to the filtrate containing Cu and Zn after recovering the goethite is adjusted to pH=5;
[0056] d, put into D-405 resin column, carry out Cu, Zn adsorption for 2 hours;
[0057] e. Wash the D-405 resin after absorbing Cu and Zn with deionized water to remove impurities, and desorb zinc with 0.2mol / L sulfuric acid solution;
[0058] f. Desorb Cu with 1.0mol / L sulfuric acid solution on the D-405 resin after desorbing Zn;
[0059] g. Adjust the desorbed zinc sulfate solution to pH=5.0, heat to evaporate→concentrate→crystallize, heat and evaporate to a density of 1.54g / mL, stop heating, and c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com