Composition for treating infection and method
A composition and virus infection technology, applied in the direction of drug combination, pharmaceutical formula, and resistance to vector-borne diseases, etc., can solve the problem of not clearing virions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
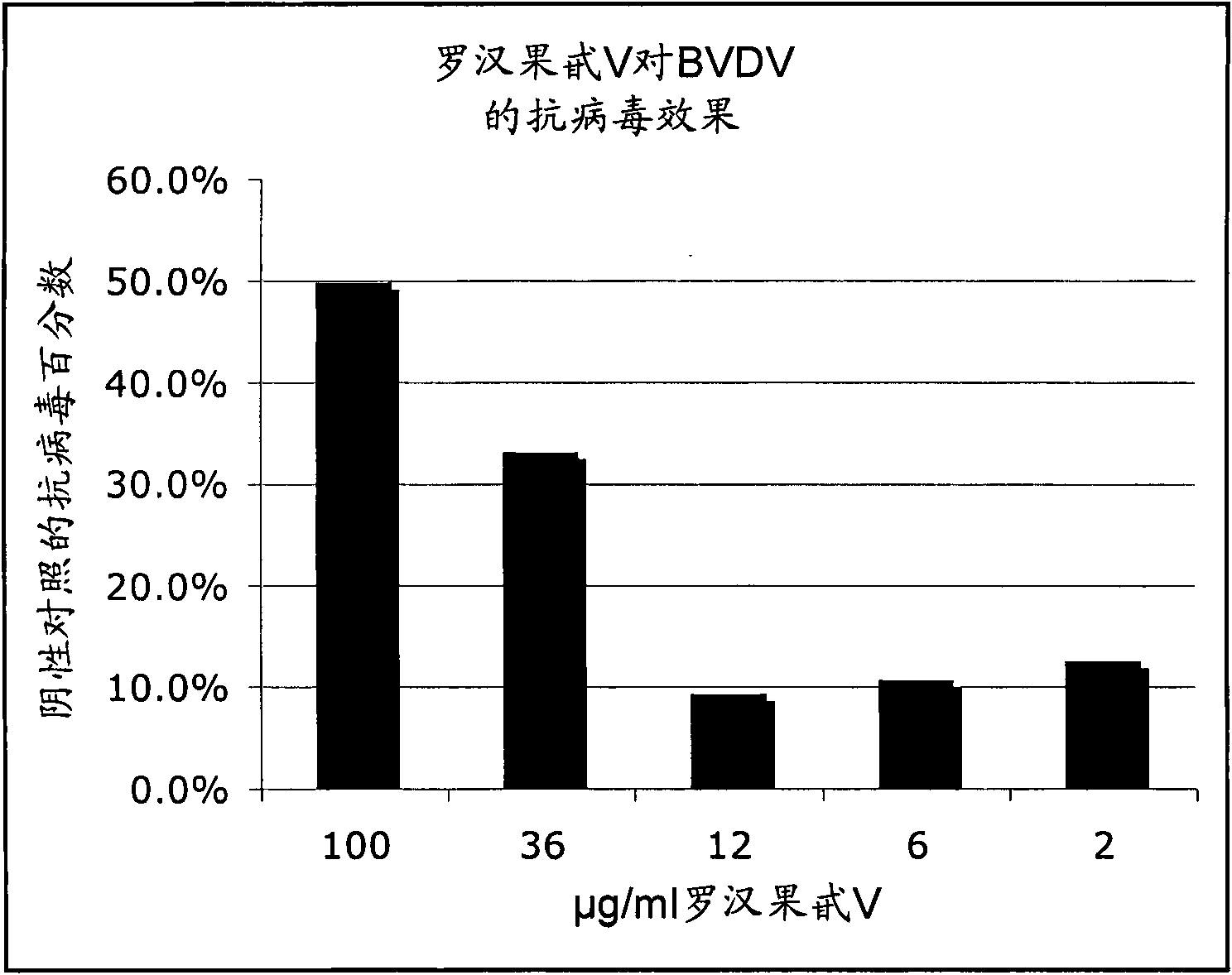
[0159] Example 1: Antiviral activity of triterpene glycoside mogroside V on BVDV
[0160] Materials and methods
[0161] The antiviral activity of mogroside V was tested against bovine viral diarrhea virus (BVDV), strain NADL. BVDV is a flavivirus of the same family as the human hepatitis C virus. Hepatitis C cannot grow in tissue culture, so BVDV was used as a model virus due to the structural similarities between the two viruses. Titrate the virus and determine the 10% of stock virus -4 Dilute to achieve a suitable inoculum for antiviral assays.
[0162] Mogroside V can be stored at room temperature and is water soluble. Mogroside V was independently tested over a wide concentration range (6.25-3600 μg / mL). Mogroside V was tested in two experiments, one at 100, 50, 25, 12.5 and 6.25 μg / mL and the other at 3600, 1200, 400, 133.3 μg / mL and 44.4 μg / mL.
[0163] Bos Taurus (BT) turbinate cells were used as host cells for the virus. After plating the cells and allowing o...
Embodiment 2
[0183] Example 2: Triterpene glycoside mogroside V on BVDV when combined with fluvastatin antiviral activity
[0184] Materials and methods
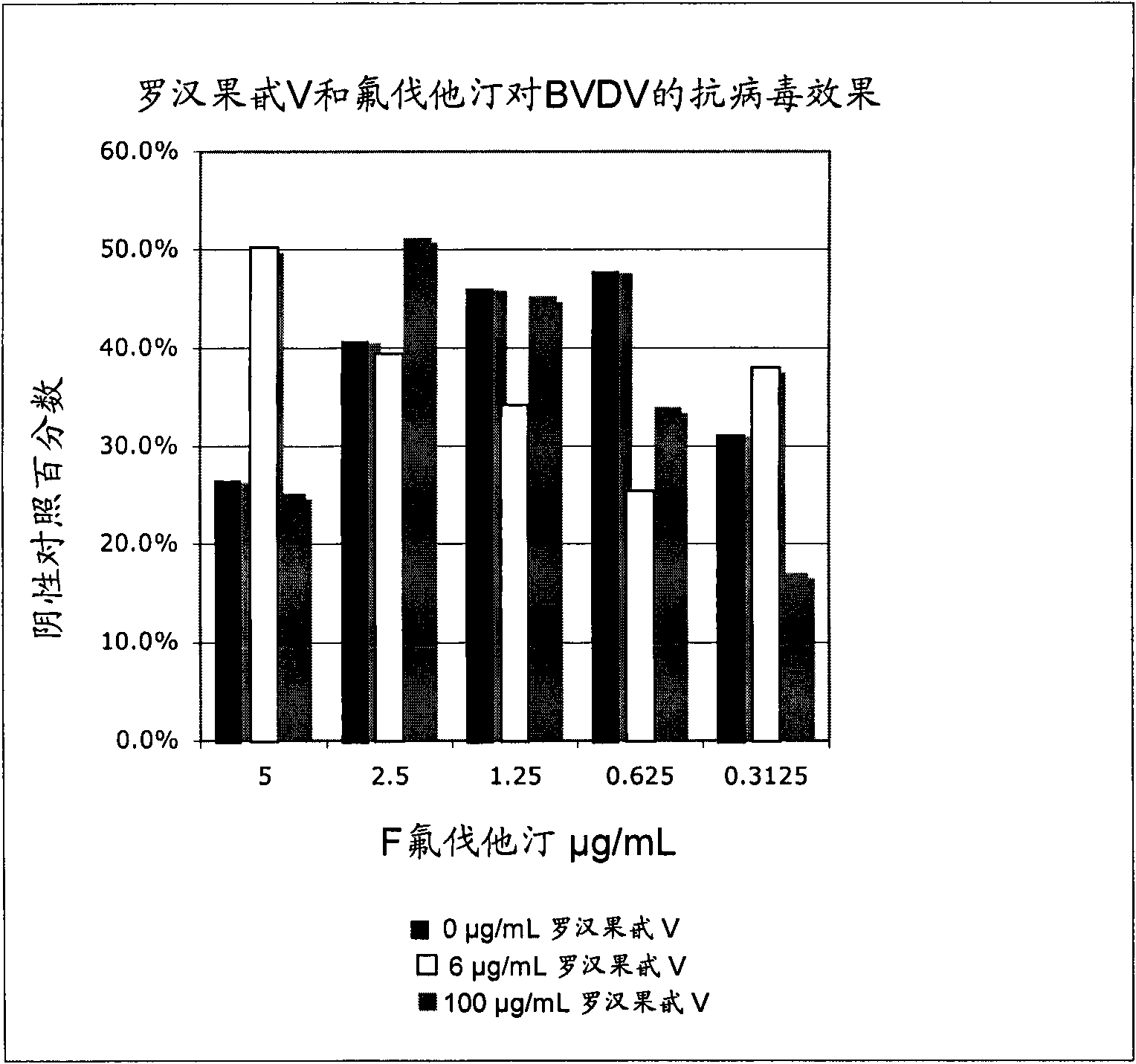
[0185] Materials and methods were similar to those outlined in Example 1. Fluvastatin must be stored between 0°C and 5°C and be water soluble. Mogroside V and fluvastatin were jointly tested, in which mogroside V had two concentrations: an effective dose (100 μg / mL) and a dose below its effective range (6 μg / mL), while fluvastatin had Within 5 doses (0.313, 0.625, 1.25, 2.5, and 5.0 μg / mL).
[0186] result
[0187] Studies have shown that fluvastatin extends the lower limit of the effectiveness of mogroside V. Especially, in the case of using 6 μg / mL mogroside V, the inhibition index of fluvastatin at 5 μg / mL and 0.3125 μg / mL was greatly improved compared with that of fluvastatin alone ( figure 2 ).
[0188] Table 3 shows the percentage and inhibition index of the negative control of various concentrations of mogroside V and...
Embodiment 3
[0197] Example 3. Antiviral activity of mogroside IV against BVDV
[0198] Materials and methods
[0199] The antiviral potency and cytotoxicity of five test compounds against bovine viral diarrhea virus (BVDV) were evaluated. BVDV is a flavivirus belonging to the same family as the human hepatitis C virus. Hepatitis C virus cannot grow in tissue culture, therefore, BVDV was used as a model virus due to the structural similarities between these two viruses. The five test compounds were evaluated in a CPE (virus-induced cytopathic effect)-inhibition assay using the BVDV strain NADL in Madin-Darby bovine kidney (Madin-Darby bovine kidney; MDBK) cells.
[0200] drug preparation
[0201] The five test compounds were supplied as dry powders and dissolved in molecular quality sterile water to obtain 20 mg / mL stock solutions listed in Table 4. All compounds were evaluated in six half log serial dilutions with a high test concentration of 100 μg / mL. Recombinant interferon a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com