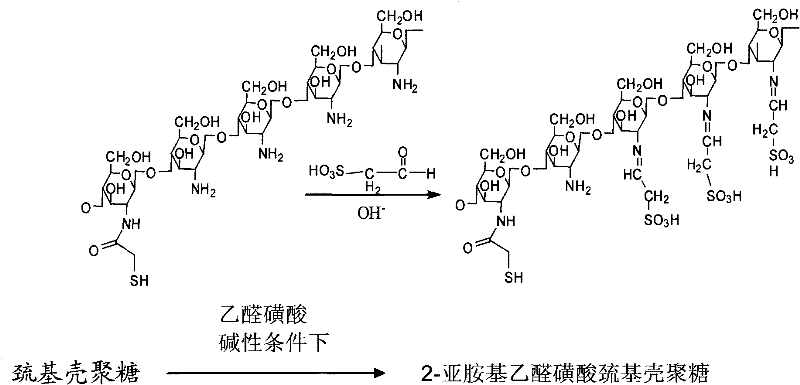
Sulfonation modification process of thio chitosan
A technology of sulfonation of chitosan, which is applied in the field of sulfonation modification process of mercapto chitosan at the N atom position, can solve the problems of short drug release time, short sustained release time, and large fluctuation of drug concentration, and achieve sulfonation High modification ratio, stable drug release, and sustained release time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
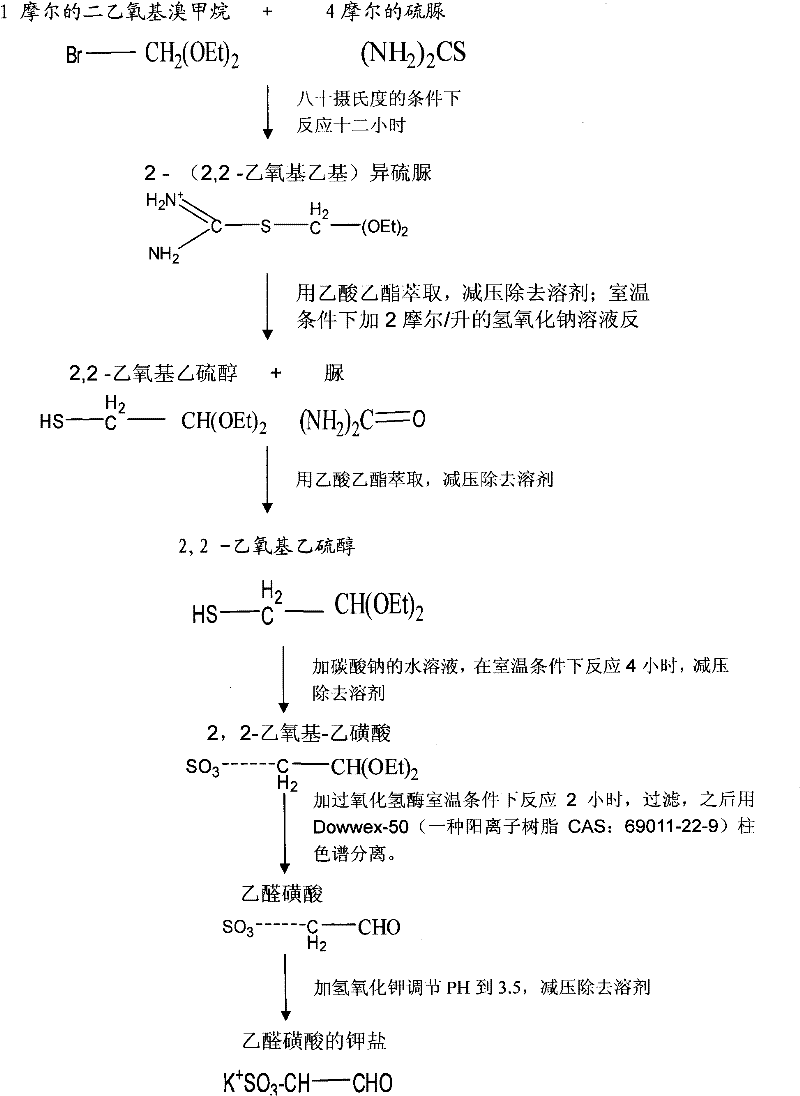
[0016] Take 18.4g of diethoxybromomethane and 30.4g of thiourea in a water bath at 80°C for 12 hours of heating reaction, the product is extracted with 50ml of ethyl acetate, and the solvent ethyl acetate is removed under reduced pressure; then add 2 mol / liter of hydroxide 0.6ml of sodium solution, react at room temperature for 3 hours, then extract with 50ml of ethyl acetate, remove the solvent ethyl acetate under reduced pressure; then add 10ml of 0.1mol / L sodium bicarbonate solution, react at room temperature for 4 hours, Remove the solvent water under reduced pressure, add 2.3g catalase to catalyze the reaction at room temperature for 2 hours, filter with filter paper, remove the filtrate, and then pass through Dowwex-50 (a cationic resin CAS: 69011-22-9) ion exchange column Carry out separation and purification, and adopt 1mol / L hydrochloric acid elution, adjust pH value to 3.5 with potassium hydroxide solution, remove solvent water under reduced pressure to obtain the pot...
Embodiment 2
[0018] Take 18.4g of diethoxybromomethane and 30.4g of thiourea in a water bath at 80°C for 12 hours of heating reaction, the product is extracted with 50ml of ethyl acetate, and the solvent ethyl acetate is removed under reduced pressure; then add 2 mol / liter of hydroxide 0.6ml of sodium solution, react at room temperature for 3 hours, then extract with 50ml of ethyl acetate, remove the solvent ethyl acetate under reduced pressure; then add 10ml of 0.1mol / L sodium bicarbonate solution, react at room temperature for 4 hours, Remove the solvent water under reduced pressure, add 2.3g catalase to catalyze the reaction at room temperature for 2 hours, filter with filter paper, remove the filtrate, then pass through Dowwex-50 ion exchange column for separation and purification, and use 1mol / L hydrochloric acid to elute , adjust the pH value to 3.5 with potassium hydroxide solution, and remove the solvent water under reduced pressure to obtain 12.4 g of potassium salt of acetaldehyde...
Embodiment 3
[0020] Take 18.4g of diethoxybromomethane and 30.4g of thiourea in a water bath at 80°C for 12 hours of heating reaction, the product is extracted with 50ml of ethyl acetate, and the solvent ethyl acetate is removed under reduced pressure; then add 2 mol / liter of hydroxide 0.6ml of sodium solution, react at room temperature for 3 hours, then extract with 50ml of ethyl acetate, remove the solvent ethyl acetate under reduced pressure; then add 10ml of 0.1mol / L sodium bicarbonate solution, react at room temperature for 4 hours, Remove the solvent water under reduced pressure, add 2.3g catalase to catalyze the reaction at room temperature for 2 hours, filter with filter paper, remove the filtrate, then pass through Dowwex-50 ion exchange column for separation and purification, and use 1mol / L hydrochloric acid to elute , adjust the pH value to 3.5 with potassium hydroxide solution, and remove the solvent water under reduced pressure to obtain 12.4 g of potassium salt of acetaldehyde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com