Method for removing alanine in reaction system for preparing neutral amino acid
A neutral amino acid and reaction system technology, applied in the field of bioengineering, can solve the problems of reducing purification costs, reducing purification steps, difficult to remove alanine, etc., and achieve the effect of increasing yield, simplifying separation, purification and refining process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
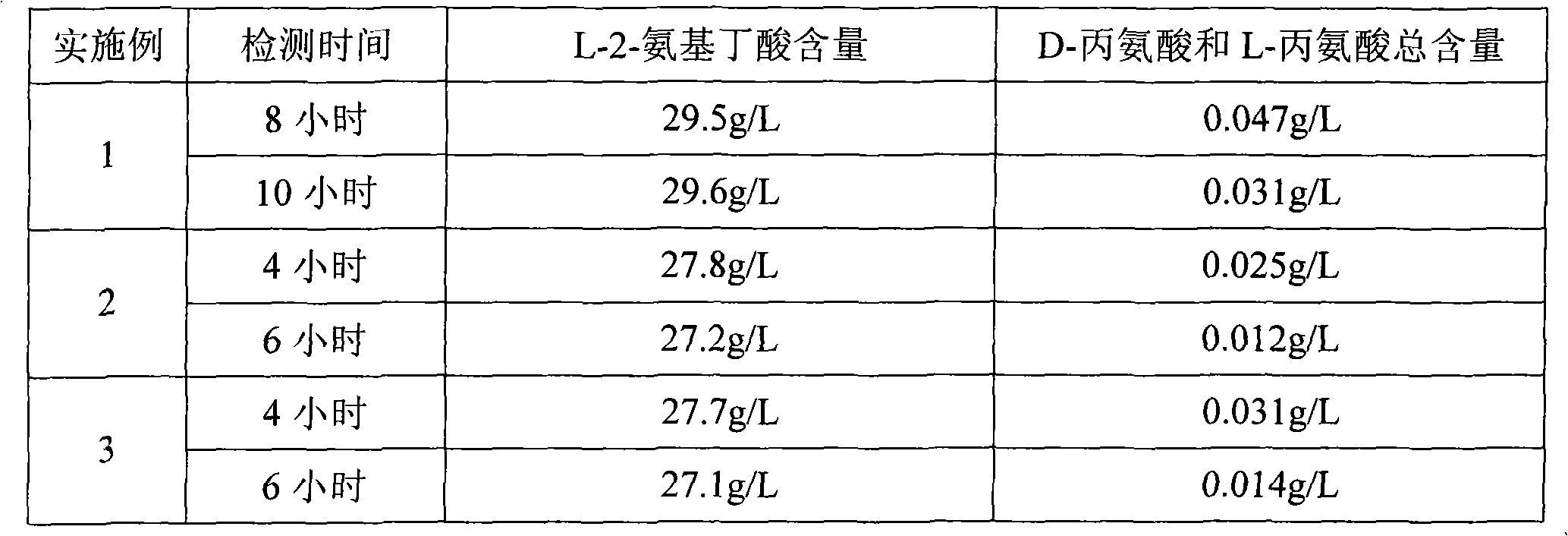
[0023] Examples 1-3 , remove L-alanine in L-2-aminobutyric acid solution
[0024] To 500ml of a solution containing L-2-aminobutyric acid 30g / L and L-alanine 5g / L, alanine racemase and D- Amino acid oxidase, then carry out the conversion reaction with the conditions shown in Table 1, and detect the content of L-2-aminobutyric acid and alanine in the reaction solution respectively, and the detection results are shown in Table 2. Embodiment 1-3 The alanine racemase used in the method is respectively alanine racemase derived from Bacillus subtilis; the D-amino acid oxidase used is the D-amino acid oxidase derived from Rhodotorula, Trichoderma and Trichoderma, respectively, Wherein, the D-amino acid oxidase in Example 3 was the immobilized Triceratops D-amino acid oxidase purchased from Hunan Flegre Biotechnology Co., Ltd.; the catalase added in Example 2-3 was purchased from Zaozhuang City Jie NovoBio Enzyme Co., Ltd.
[0025] Table 1. Transformation conditions
[0026] ...
Embodiment 4-5
[0030] Example 4-5 , remove alanine in L-tert-leucine solution and L-valine solution
[0031] Add alanine to 100ml of the solution containing L-tert-leucine 30g / L and L-alanine 6g / L, and 500ml of the solution containing L-valine 30g / L and L-alanine 4g / L, respectively Acid racemase, amino acid oxidase and 5000U / L catalase, then add 20% hydrogen peroxide solution at a rate of 0.5ml / min, the temperature is controlled at 25°C, the pH is maintained at 8.0, and the stirring is at 200rpm. The conversion reaction was carried out under the condition of the speed, and then respectively 4 hours and 6 hours after the reaction, the content of the target amino acid and alanine in the reaction solution was detected, and the detection results were as shown in Table 3, wherein, in the embodiment 4-5 The alanine racemase used is 3000U / L alanine racemase derived from Bacillus subtilis; the amino acid oxidase used is 2000U / L D-amino acid oxidase derived from Rhododendron, 2000U / L source D-amin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com