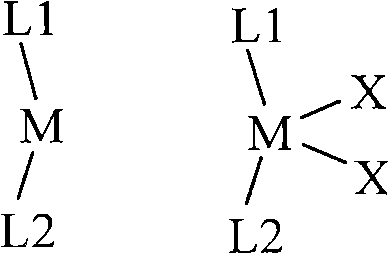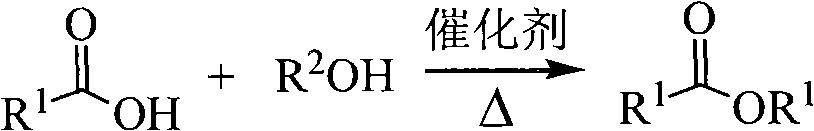Metallocene compound and application of byproduct thereof in catalytic esterification reaction
A metallocene compound, catalytic esterification technology, applied in the direction of organic compound / hydride / coordination complex catalyst, physical / chemical process catalyst, catalyst carrier, etc., can solve the problems that have not been seen, and achieve the production route environmental protection, product quality The effect of high purity and low dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthesis of Butyl Acetate Catalyzed by Ferrocene
[0024] Weigh 28.2mg of ferrocene, measure 18mL of acetic acid and 30mL of n-butanol, add all into a 200mL single-necked flask, add a few zeolite, install a water separator, heat to reflux for 45 minutes, cool the liquid in the water separator to the flask The medium and liquid are combined, washed with equal amount of water and separated, the organic phase is taken, dried with anhydrous magnesium sulfate, distilled at atmospheric pressure, collected 125-127°C fractions to obtain 22.91g of colorless and transparent liquid product, yield 66%, gas chromatography detection The purity is 98%.
Embodiment 2
[0026] Synthesis of Butyl Acetate Catalyzed by Titanocene
[0027] Weigh 12.2mg of titanocene, measure 6mL of acetic acid and 10mL of n-butanol into a 100mL single-necked flask, add zeolite, install a water trap, heat and reflux for 30 minutes, cool, combine the liquid in the water trap with the liquid in the bottle, After washing with equal amount of water, the liquid was separated, the organic phase was taken, dried with anhydrous magnesium sulfate, distilled under normal pressure, and collected 125-127°C fractions to obtain 10.44g of a colorless and transparent liquid product with a yield of 90% and a purity of 99% by gas chromatography.
Embodiment 3
[0029] Synthesis of Butyl Acetate Catalyzed by Zirconocene
[0030] Weigh 15.3mg of zirconocene, 6mL of acetic acid and 10mL of n-butanol into a 100mL single-neck flask, add zeolite, install a water separator, heat and reflux for 40 minutes, after cooling, combine the liquid in the water separator with the liquid in the bottle, and equal amounts After washing with water, the liquid was separated, the organic phase was taken, dried with anhydrous magnesium sulfate, and distilled under normal pressure. The 125-127°C fraction was collected to obtain 9.28 g of a colorless and transparent liquid product with a yield of 80% and a purity of 99% by gas chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com