Internal lumen inner bracket with bilateral medicine-releasing performance and preparation method thereof
A technology of internal stents and drugs, applied in the field of medical devices, can solve the problems of local bacterial infection, loss of open lumen, failure to prevent tumor longitudinal overgrowth and stent compressive restenosis, etc., and achieve the effect of preventing adhesion and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
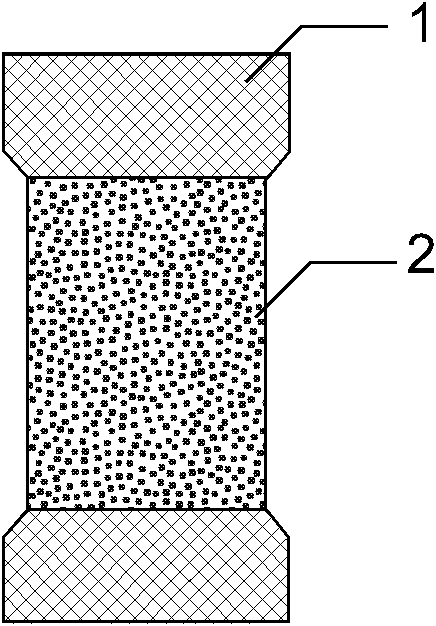
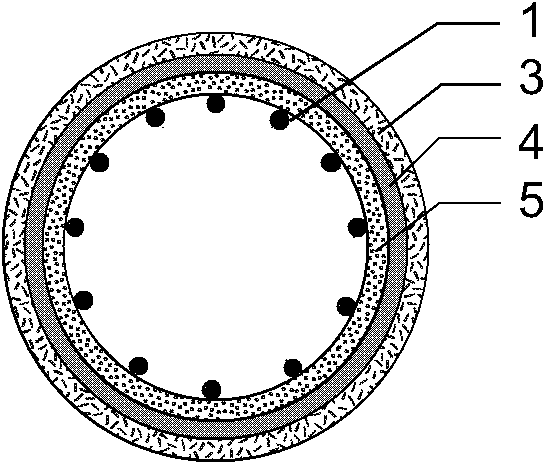
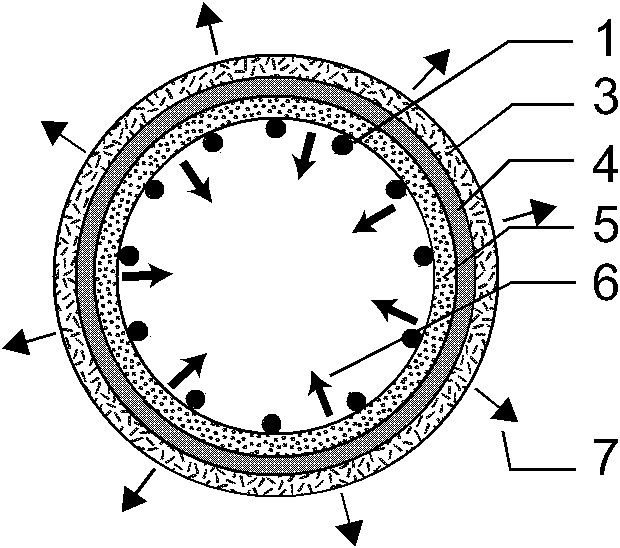
[0039] Such as figure 1 , 2 , 3, the present invention consists of a bare metal stent 1 and a coating 2, which is characterized in that the coating 2 includes three parts: an outer drug sustained-release layer 3, an isolation layer 4, and an inner drug sustained-release layer 5, and the coating 2 wraps On the outer surface of the bare metal stent 1, the outer drug sustained-release layer 3 is located at the outermost side of the stent and unidirectionally releases anti-tumor drugs toward the outer side of the stent 7, and the inner drug sustained-release layer 5 is located at the innermost side of the stent and toward the inner side of the stent lumen 6 One-way release of antibiotic drugs, the isolation layer 4 is located between the outer drug sustained release layer 3 and the inner drug sustained release layer 5 to prevent drug penetration. The outer drug sustained release layer 3 is composed of polyethylene vinyl acetate and natural plant extract class anticancer drug pacl...
Embodiment 2
[0048] Such as figure 1 , 4 , 5, the present invention consists of a bare metal stent 1 and an envelope 2, which is characterized in that the envelope 2 includes three parts: an outer drug sustained-release layer 3, an isolation layer 4, and an inner drug sustained-release layer 5, and the bare metal stent 1 is embedded In the isolation layer 4 of the envelope 2, the outer drug sustained release layer 3 is located at the outermost side of the stent and releases antitumor drugs unidirectionally toward the outer side of the stent 7, and the inner drug sustained release layer 5 is located at the innermost side of the stent and toward the stent lumen. The inner direction 6 releases antibiotic drugs unidirectionally, and the isolation layer 4 is located between the outer drug sustained-release layer 3 and the inner drug sustained-release layer 5 to prevent drug penetration. The outer drug sustained release layer 3 is composed of polyacrylate and irinotecan; the inner drug sustaine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com