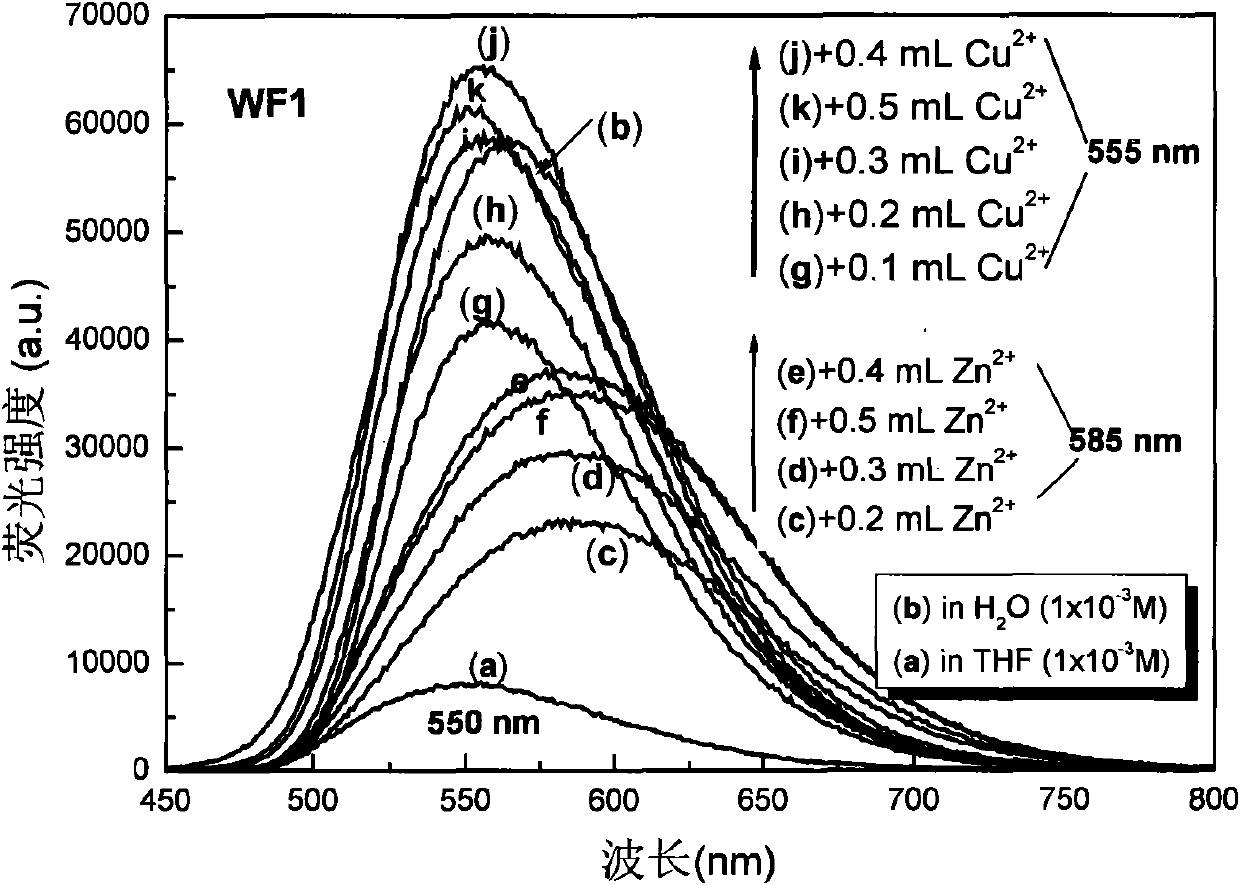
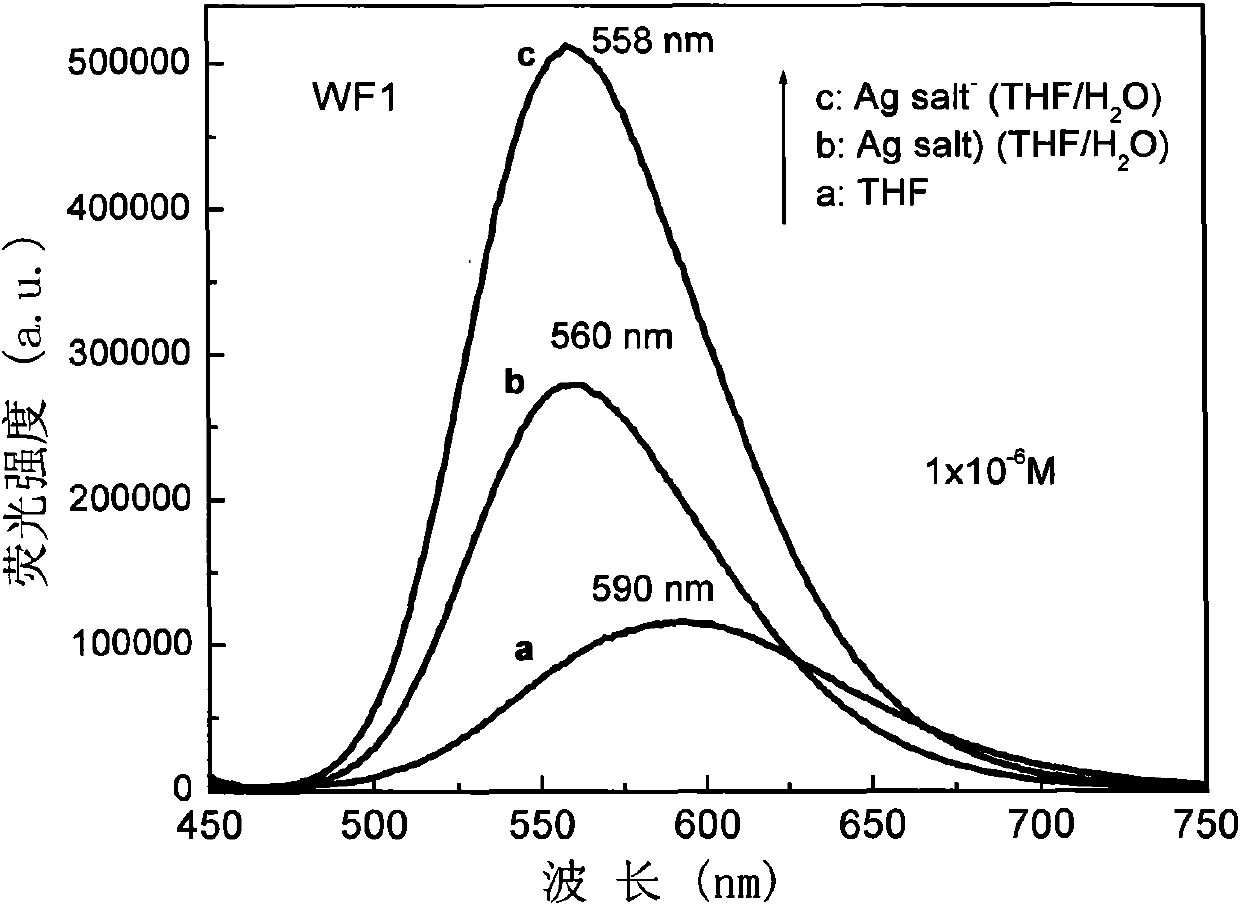
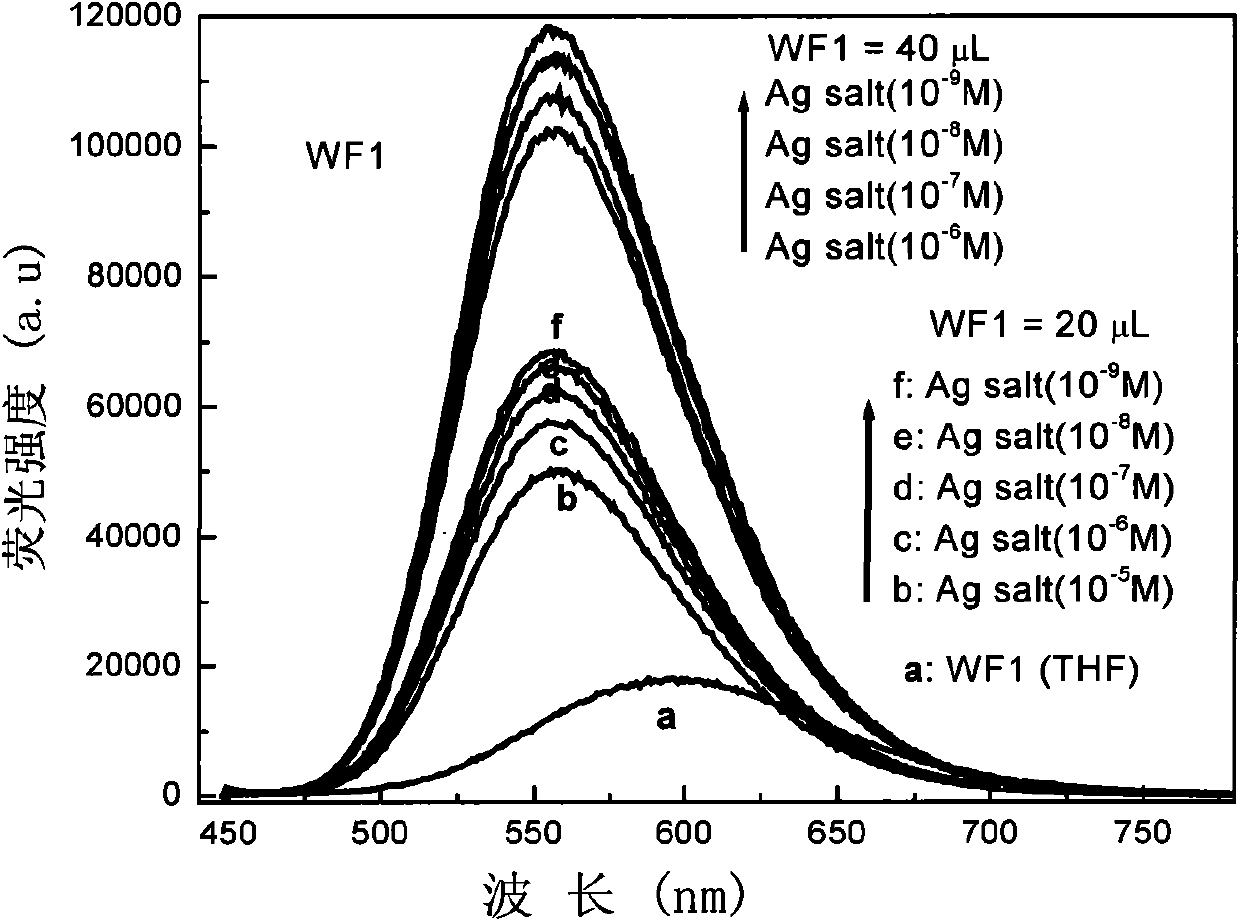
Pyridine chalcone derivative with aggregation sate fluorescence enhancement and two-photon fluorescence characteristics
A technology of pyridylchalcone and two-photon fluorescence, which is applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of limited application, few types of materials for enhanced fluorescence in aggregated state, etc., and achieve simple preparation process and easy Purification and industrialization, the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 (WF1): Synthesis of (E)-N,N-diphenyl-4-(2-acryloylpyridyl)aniline.
[0039] (1) Synthesis of N, N-diphenylaminobenzaldehyde: Weigh equimolar amounts of triphenylamine and DMF in a three-necked flask, slowly drop in excess phosphorus oxychloride in an ice bath, and heat up to reflux after dropping for 1 After cooling, slowly pour it into ice water, neutralize it with aqueous sodium hydroxide solution, stir vigorously and let it stand, and filter to obtain the crude product. Finally, light yellow crystals were obtained by vacuum sublimation, which was N, N-diphenylaminobenzaldehyde, with a yield of 89.5%;
[0040] (2) Add self-made N,N-diphenylaminobenzaldehyde (10.8mmol, 2.95g) into 20mL of alcohol (such as methanol, ethanol, etc.), and then add 2-acetylpyridyl (10.8mmol, 1.31 g) and 8mL saturated lye (such as potassium carbonate, NaOH, KOH aqueous solution); stirred at room temperature for 24 hours, filtered the crude product and then rinsed several times wit...
Embodiment 2
[0049] Example 2 (WW1): Synthesis of (E)-N-[4-(2-acryloylpyridyl)]phenylcarbazole.
[0050] (1) Synthesis of N-carbazolylbenzaldehyde: Weigh equimolar amounts of carbazole and p-fluorobenzaldehyde in a three-necked flask, then add excess potassium carbonate and the amount of cetyltrimethyl bromide as catalyst Amine, heated at 136°C in DMF solvent for 3 days, cooled and slowly poured into ice water, stirred and extracted three times with DCM, concentrated to obtain a crude product, and finally obtained a yellow crystal by vacuum sublimation, which is N-carbazolylbenzaldehyde , yield 85.2%;
[0051] (2) Using a method similar to that in Example 1, it is only necessary to change the N,N-diphenylaminobenzaldehyde in step 2 into N-carbazolylbenzaldehyde according to the obtained compound, and the yield is 56.3%;
[0052] (3) Analysis of the obtained compound: yellow, melting point: 200°C, decomposition temperature: 210°C.
[0053] MS (EI), found: 374.1; calculated: 374.14.
[00...
Embodiment 3
[0059] Example 3 (WW2): Synthesis of (E)-N-ethyl-[3-(2-acryloylpyridyl)]carbazole.
[0060] (1) N-ethyl-3-carbazole formaldehyde: the DMF of 3ml is joined in the flask that fills 3.5g (18mmol) N-ethylcarbazole, ice bath and N 2 Under atmosphere, add 0.09 mol of POCl dropwise 3 . After the dropwise addition was completed, the temperature was raised to 90°C to react for 24 hours, cooled to room temperature, the reaction solution was slowly poured into ice water, neutralized with dilute acid, extracted with dichloromethane, anhydrous MgSO 4 After drying, it was purified by silica gel column packing to obtain 2.4 g of N-ethyl-3-carbazole formaldehyde with a yield of 63%.
[0061] (2) The synthetic method is similar to Example 1, only need to change the N,N-diphenylaminobenzaldehyde in step (2) into N-ethyl-3-carbazole formaldehyde according to the compound obtained, and the productive rate is 64.3 %.
[0062] (3) Analysis of the obtained compound: yellow, with a decomposition ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com