Process for preparing bosutinib
A preparation process, the technology of bosutinib, applied in the chemical and pharmaceutical field, can solve the problems of harshness, increase the reaction route, and low yield, and achieve the effect of short route, high yield and mild cyclization conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
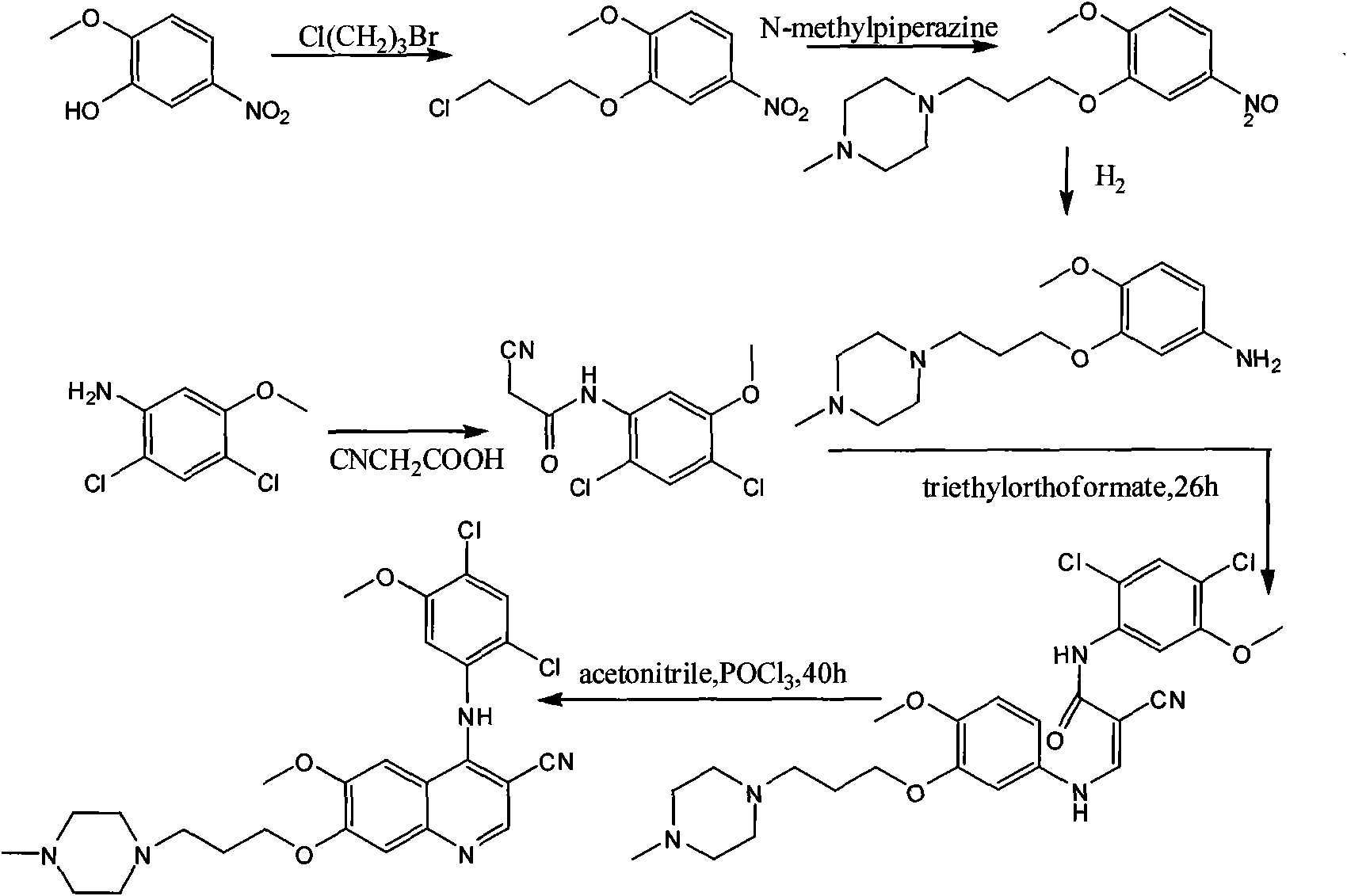
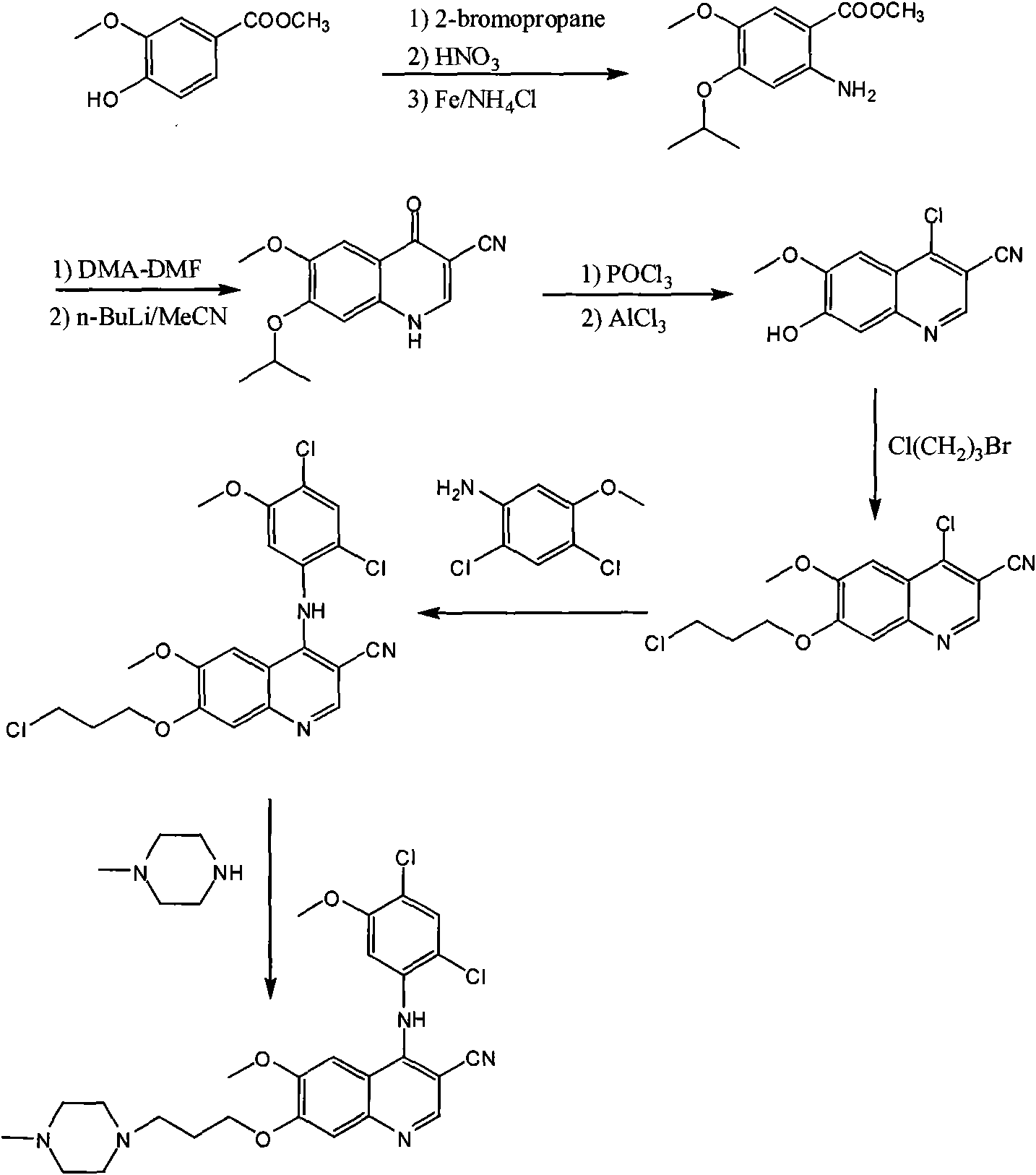
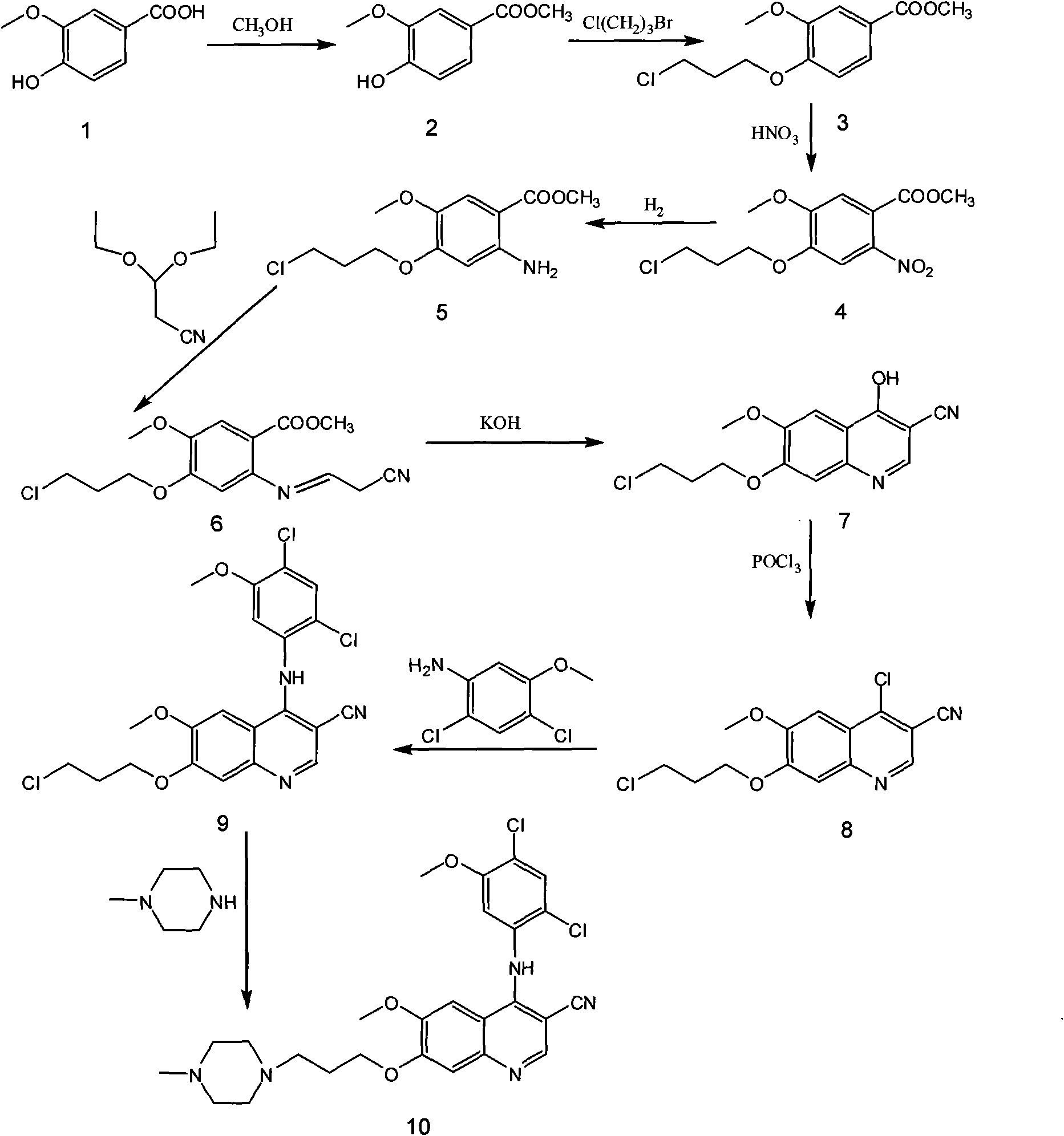
[0017] The preparation process of bosutinib, the preparation steps are: take 4-hydroxy-3-methoxybenzoic acid as raw material, react with methanol to form an ester, and obtain methyl 4-hydroxy-3-methoxybenzoate; Hydroxy-3-methoxybenzoic acid methyl ester reacts with 1-bromo-3-chloropropane to obtain 4-(3-chloropropoxy)-3-methoxybenzoic acid methyl ester after alkylation; 4 The molar ratio of -hydroxy-3-methoxymethylbenzoate to 1-bromo-3-chloropropane is 1:1, and the reaction temperature is 50°C; 4-(3-chloropropoxy)-3-methoxy Methyl benzoate was nitrated to obtain methyl 4-(3-chloropropoxy)-5-methoxy-2-nitrobenzoate, 4-(3-chloropropoxy)-3-methoxy The molar ratio of methyl benzoate and fuming nitric acid is 1:1, and the reaction temperature is 40°C; methyl 4-(3-chloropropoxy)-5-methoxy-2-nitrobenzoate is obtained by reduction 4-(3-chloropropoxy)-5-methoxy-2-aminobenzoic acid methyl ester, the reducing agent used is iron powder, zinc powder or palladium carbon, wherein 4-(3-chlor...
Embodiment 2
[0019]The preparation process of bosutinib, the preparation steps are: take 4-hydroxy-3-methoxybenzoic acid as raw material, react with methanol to form an ester, and obtain methyl 4-hydroxy-3-methoxybenzoate; Hydroxy-3-methoxybenzoic acid methyl ester reacts with 1-bromo-3-chloropropane to obtain 4-(3-chloropropoxy)-3-methoxybenzoic acid methyl ester after alkylation; 4 The molar ratio of -hydroxy-3-methoxybenzoic acid methyl ester to 1-bromo-3-chloropropane is 1:3, and the reaction temperature is 80°C; 4-(3-chloropropoxy)-3-methoxy Methyl benzoate was nitrated to obtain methyl 4-(3-chloropropoxy)-5-methoxy-2-nitrobenzoate, 4-(3-chloropropoxy)-3-methoxy The molar ratio of methyl benzoate and fuming nitric acid is 1:2, and the reaction temperature is 60°C; methyl 4-(3-chloropropoxy)-5-methoxy-2-nitrobenzoate is obtained by reduction 4-(3-chloropropoxy)-5-methoxy-2-aminobenzoic acid methyl ester, the reducing agent used is iron powder, zinc powder or palladium carbon, wherein ...
Embodiment 3
[0021] The preparation process of bosutinib, the preparation steps are: take 4-hydroxy-3-methoxybenzoic acid as raw material, react with methanol to form an ester, and obtain methyl 4-hydroxy-3-methoxybenzoate; Hydroxy-3-methoxybenzoic acid methyl ester reacts with 1-bromo-3-chloropropane to obtain 4-(3-chloropropoxy)-3-methoxybenzoic acid methyl ester after alkylation; 4 The molar ratio of -hydroxy-3-methoxybenzoic acid methyl ester to 1-bromo-3-chloropropane is 1:5, and the reaction temperature is 110°C; 4-(3-chloropropoxy)-3-methoxy Methyl benzoate was nitrated to obtain methyl 4-(3-chloropropoxy)-5-methoxy-2-nitrobenzoate, 4-(3-chloropropoxy)-3-methoxy The molar ratio of methyl benzoate to fuming nitric acid is 1:3, and the reaction temperature is 80°C; methyl 4-(3-chloropropoxy)-5-methoxy-2-nitrobenzoate is obtained by reduction 4-(3-chloropropoxy)-5-methoxy-2-aminobenzoic acid methyl ester, the reducing agent used is iron powder, zinc powder or palladium carbon, wherein...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com