Medicament for resisting influenza and preparation method and application thereof
An anti-influenza and pharmaceutical preparation technology, applied in the field of anti-influenza, can solve the problems of ineffectiveness and high toxicity of vaccines and targeted drugs, and achieve the effects of easy promotion, rational selection of prescriptions, low economic cost and resource cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
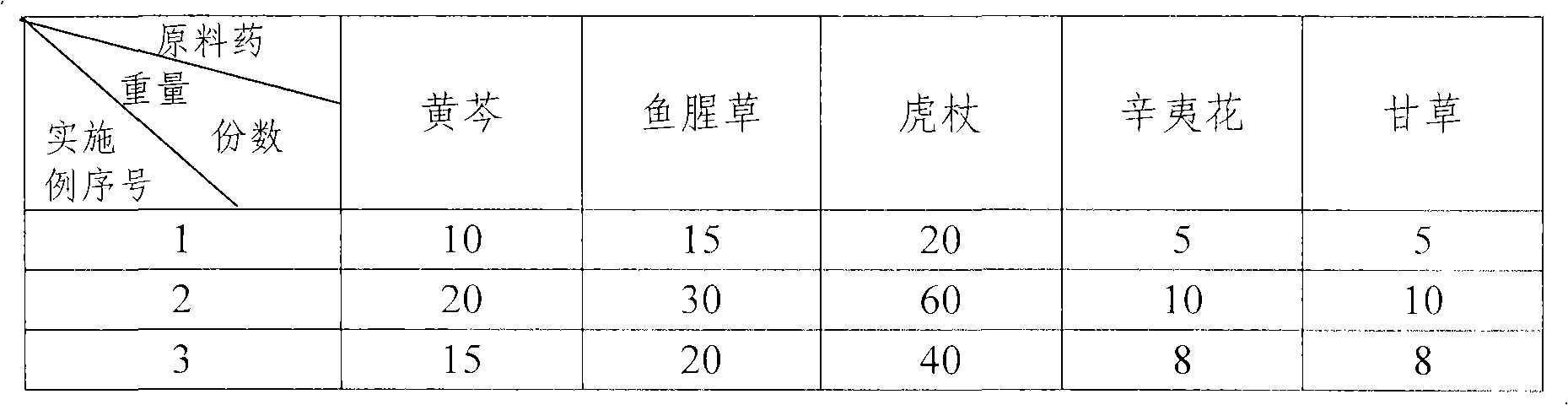
Embodiment 1-4
[0030] The preparation method of embodiment 1-4:
[0031] 1) Weigh Houttuynia cordata according to the amount converted in parts by weight shown in the above table, properly pulverize, sieve, place in a volatile oil extraction device, add 10 times the amount of water, soak in warm for 0.5h, heat, steam distill, Distillate was collected after 4h. The distillate was extracted three times with ether, the extract was dried with anhydrous sodium sulfate for 24 hours, and then filtered. The filtrate was evaporated with a rotary evaporator to remove the ether, and the volatile oil was collected. The volatile oil appeared light yellow-green. The residue was decocted twice again, adding 5 times the amount of water each time, decocting for 1 hour, filtering, combining the filtrates for later use, and discarding the residue.
[0032] 2) Scutellaria baicalensis, Polygonum cuspidatum, Xinyi flower and licorice were weighed according to the amounts converted in parts by weight shown in the...
Embodiment 5
[0034] The preparation method of embodiment 5:
[0035] 1) Weigh Scutellaria baicalensis, Polygonum cuspidatum, Magnolia magnolia and Radix Glycyrrhizae according to the amounts converted in parts by weight shown in the above table. Appropriately pulverize, sieve, add 10 times the amount of water, decoct twice for 2 hours each time, filter the extract, and combine the filtrates, each ml is equivalent to 0.1 g of raw material medicine.
[0036] 3) Preparation process: add an appropriate amount of sucrose to the combined filtrate, and then divide into 10 ml bottles to obtain oral preparations.
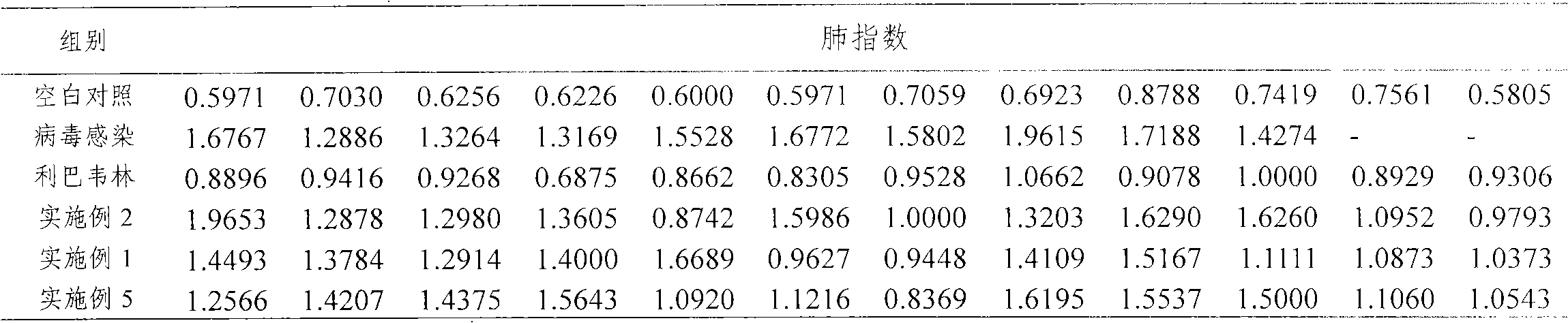
experiment example
[0038] 1. Materials and methods
[0039] 1. Viruses
[0040] Influenza virus murine lung-adapted strain FM1 (type A H1N1 virus) was provided by the Institute of Virology, Chinese Academy of Preventive Medicine. Passage chicken embryos, test in BSL-3 (Biosafety Laboratory Level 3), subpackage, and store at -80°C.
[0041] 2. Experimental animals
[0042] 72 BALB / c mice weighing 20-22 g were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., and were bred under clean-grade conditions in the animal room of our institute.
[0043] 3. Experimental drugs
[0044] The experimental medicines were the medicines of Examples 1, 2 and 5. The positive control drug was commercially available ribavirin (0.15 g once for adults, 3 times a day for 7 consecutive days) approved by SFDA (Sichuan Baili Pharmaceutical lot: 081017).
[0045] 4. Experimental method
[0046] Mice are divided into 6 groups at random and are blank control group, virus infection group, po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com