Method for measuring arsenic by iodine solution absorption method
A technology of iodine solution and absorption method, which is applied to the measurement of color/spectral characteristics, material analysis by observing the influence of chemical indicators, and analysis by making materials undergo chemical reactions, etc. It can solve the problems of toxic waste liquid and achieve Save reagent costs, avoid the use of toxic and harmful reagents, and reduce environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
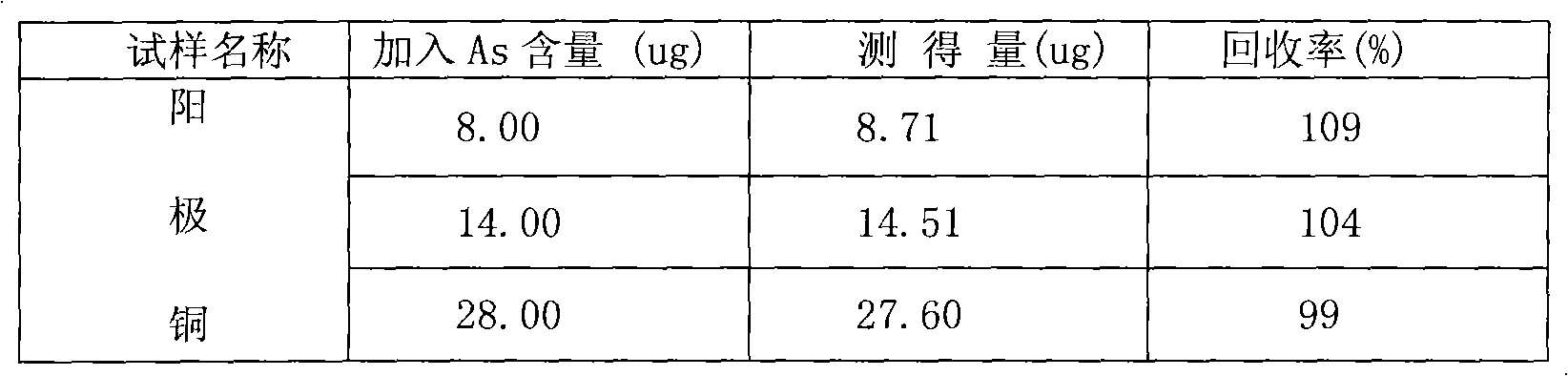
[0027] Take the copper concentrate sample, accurately weigh 0.5000g sample, add 15ml (1+1) sulfuric acid and 5ml nitric acid to a 100ml beaker, cover with a watch glass, dissolve at 100-200°C, and steam until the sulfuric acid is just emerging To smoke, remove to cool. Wash the watch glass and cup wall with water, without covering the watch glass, heat at 100-200°C until sulfuric acid fumes just come out, remove and cool. Transfer to a 100ml volumetric flask. Take an appropriate amount of test solution in a 250ml conical beaker, add 50ml of distilled water, add 15ml (1+1) sulfuric acid, 5ml of potassium iodide solution, mix well, add 0.5ml of tin dichloride solution, shake well, reduce to yellow and disappear, Add 5ml of tartaric acid solution and shake well. Leave it for 10 minutes, add 4 grams of arsenic-free zinc particles, quickly stuff it into a 25ml colorimetric tube with a gas tube and the other end of the tube has been inserted in advance with 10ml of absorption solu...
Embodiment 2- Embodiment 6
[0035] Weigh 5 parts of 0.5000g sample, place them in 100ml beakers respectively, add 15ml (1+1) sulfuric acid, 5ml nitric acid, cover with a watch glass, dissolve at 100-200°C, steam until sulfuric acid fumes are just emerging, remove cool down. Wash the watch glass and cup wall with water, without covering the watch glass, heat at 100-200°C until sulfuric acid fumes just come out, remove and cool. Transfer to a 100ml volumetric flask. Take an appropriate amount of test solution in a 250ml conical beaker, add 50ml of distilled water, add 15ml (1+1) sulfuric acid, add 3ml, 4ml, 5ml, 6ml, 7ml of potassium iodide solution, mix well, add tin dichloride solution 0.5 ml, shake well, restore until the yellow color disappears, add 5ml of tartaric acid, shake well. Leave it for 10 minutes, add 4 grams of arsenic-free zinc particles, quickly stuff it into a 25ml colorimetric tube with a gas tube and the other end of the tube has been inserted in advance with 10ml of absorption soluti...
Embodiment 7- Embodiment 9, comparative example 2
[0040] Embodiment 7-embodiment 9, comparative example 2-comparative example 3
[0041] Weigh 5 parts of 0.5000g sample, place them in 100ml beakers respectively, add 15ml (1+1) sulfuric acid, 5ml nitric acid, cover with a watch glass, dissolve at 100-200°C, steam until sulfuric acid fumes are just emerging, remove cool down. Wash the watch glass and cup wall with water, without covering the watch glass, heat at 100-200°C until sulfuric acid fumes just come out, remove and cool. Transfer to a 100ml volumetric flask. Take an appropriate amount of test solution in a 250ml conical beaker, add 50ml of distilled water, add 15ml (1+1) sulfuric acid, 5ml of potassium iodide solution, mix well, add 0.5ml, 1ml, 2ml, 4ml, 5ml of tin dichloride solution respectively , Shake well, reduce to yellow disappear, add 5ml of tartaric acid solution, shake well. Leave it for 10 minutes, add 4 grams of arsenic-free zinc particles, quickly stuff it into a 25ml colorimetric tube with a gas tube an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com