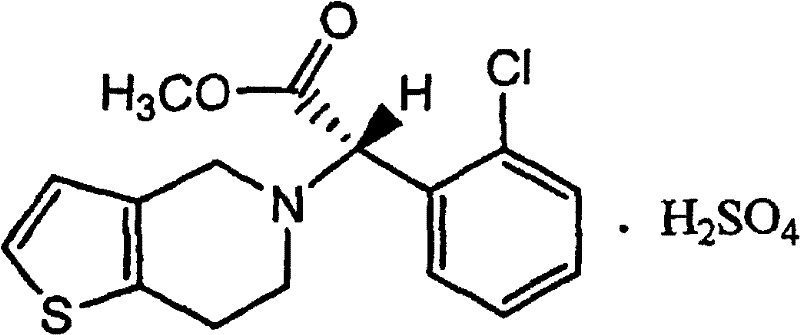
Method for synthesizing related substance C of clopidogrel hydrogen sulfate
A technology of clopidogrel bisulfate and clopidogrel hydrochloride, which is applied in the field of organic chemical synthesis, can solve the problems of high material cost, achieve short reaction time, reduce the emission of three wastes, and achieve the effects of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
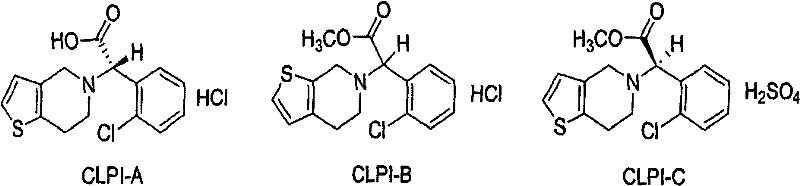
[0022] Put 35.8g (0.1mol) of clopidogrel hydrochloride, 71.6ml (twice) of ethyl acetate, and 100ml of water into the reaction flask, adjust the pH to neutral with sodium bicarbonate for dissociation, dry the obtained organic layer and add 9.3 g (0.04mol) left-handed camphorsulfonic acid, crystallized for 4 hours at 0°C, filtered to obtain the left-handed camphorsulfonate of dextro-clopidogrel, added 13.9g (0.06mol) right-handed camphorsulfonic acid in the clopidogrel mother liquor, Crystallization at -20°C for 2 hours, filtered to obtain 16.6 g of dextrocamphorsulfonate of clopidogrel, mp 184-186°C, [α] D =-56.0° HPLC 98.73%, yield 29.96%. Salt with sulfuric acid to obtain the related substance C.
Embodiment 2
[0024] Put 35.8g (0.1mol) of clopidogrel hydrochloride, 71.6ml (2 times) of ethyl acetate, and 100ml of water into the reaction flask, adjust the pH to neutral with sodium bicarbonate for freeing, dry the organic layer and concentrate it into an oily substance, add 143.2 ml (4 times) of acetone, 11.6g (0.05mol) of L-camphorsulfonic acid, crystallized at 0°C for 4 hours, filtered to obtain the L-camphorsulfonate of D-clopidogrel, and 11.6g (0.05mol) of L-camphorsulfonic acid was added to the mother liquor. Rotate camphorsulfonic acid, crystallize at -10°C for 4 hours, filter to obtain 16.9 g of D-camphorsulfonate of L-clopidogrel, mp184-186°C, [α] D =-56.0° HPLC 98.88%, yield 30.50%. Salt with sulfuric acid to obtain the related substance C.
Embodiment 3
[0026] Put 35.8g (0.1mol) of clopidogrel hydrochloride, 214.8ml (6 times) of ethyl acetate, and 100ml of water into the reaction flask, adjust the pH to neutral with sodium bicarbonate for freeing, and add 13.9g (0.06mol) after drying the organic layer Left-handed camphorsulfonic acid, crystallization at 0°C for 4 hours, filtered to obtain the left-handed camphorsulfonate of dextroclopidogrel, 9.3g (0.04mol) of right-handed camphorsulfonic acid was added to the mother liquor, crystallization at 5°C for 6 hours, filtered Obtain 17.3g of D-camphorsulfonate of L-clopidogrel, mp184-186℃, [α] D =-56.0° HPLC 99.44%, yield 31.22%. Salt with sulfuric acid to obtain the related substance C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com