Irbesartan hydrobromide and preparation method thereof
A hydrobromide, hydrobromic acid technology, applied in metabolic diseases, cardiovascular system diseases, urinary system diseases and other directions, can solve the problems such as product purity and particle size cannot be well controlled, and achieve the advantages of industrial operation, The effect of smooth surface and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
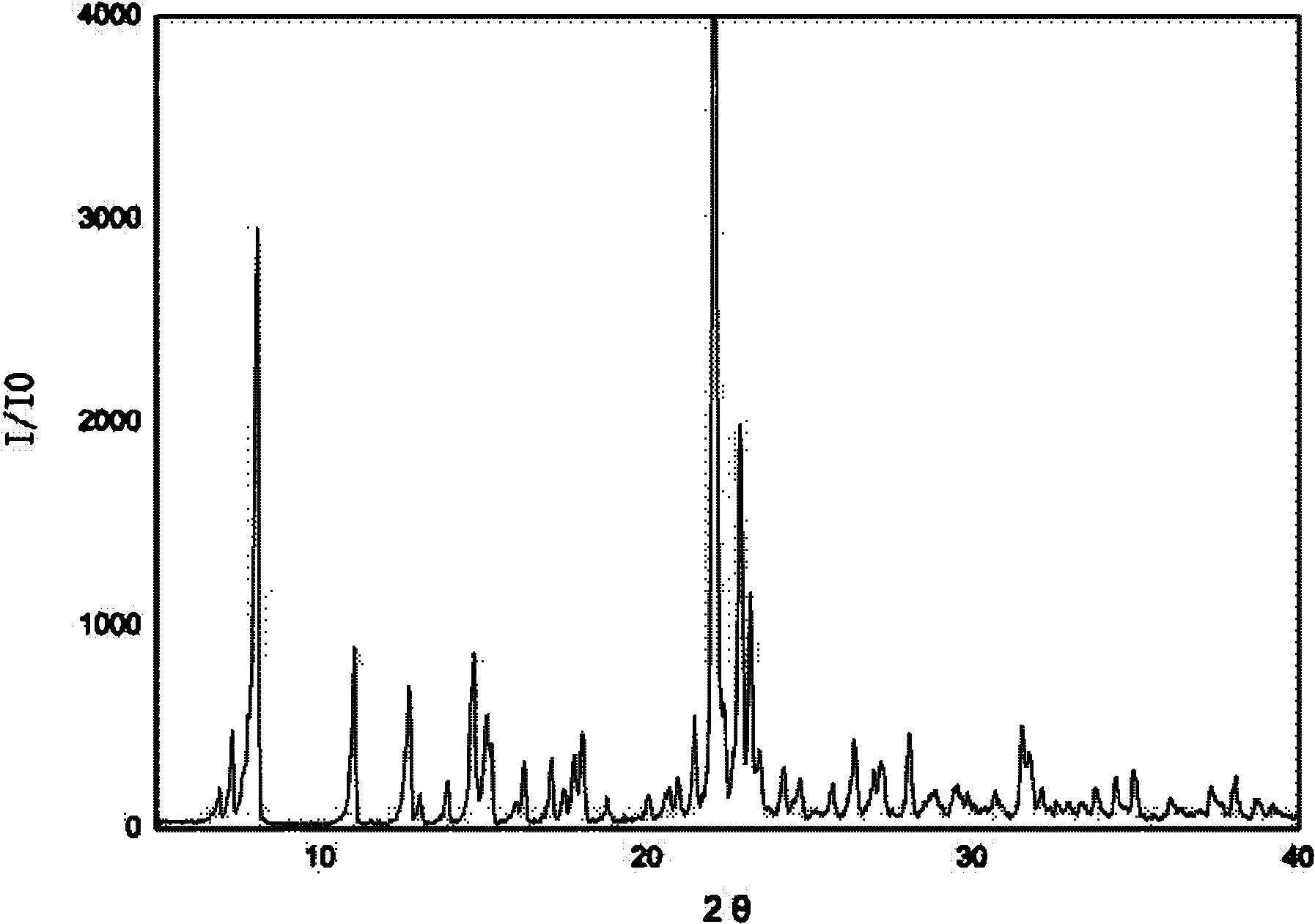
[0021] 3g of irbesartan was added to the crystallizer, and 3mL of tetrahydrofuran was added to disperse the irbesartan in a slurry form in the solvent. Add 1.78 g of 40% hydrobromic acid and stir to complete the reaction. Begin to add ethyl acetate dropwise, and the amount of ethyl acetate added is 30 mL in total. Suction filtration, ethyl acetate washing. The product was left to dry naturally at room temperature for 3 hours to obtain irbesartan hydrobromide, wherein the mass percentage of water was 5%, and the X-ray powder diffraction characteristic peak positions of the product were expressed in 2θ as: 8.06, 10.94, 12.63 , 14.74, 15.06, 22.05, 22.86, 23.16, 26.47, 31.47. There are two endothermic peaks in DSC differential scanning calorimetry, and the corresponding peak top temperatures are 139.5°C and 199.8°C, respectively.
Embodiment 3
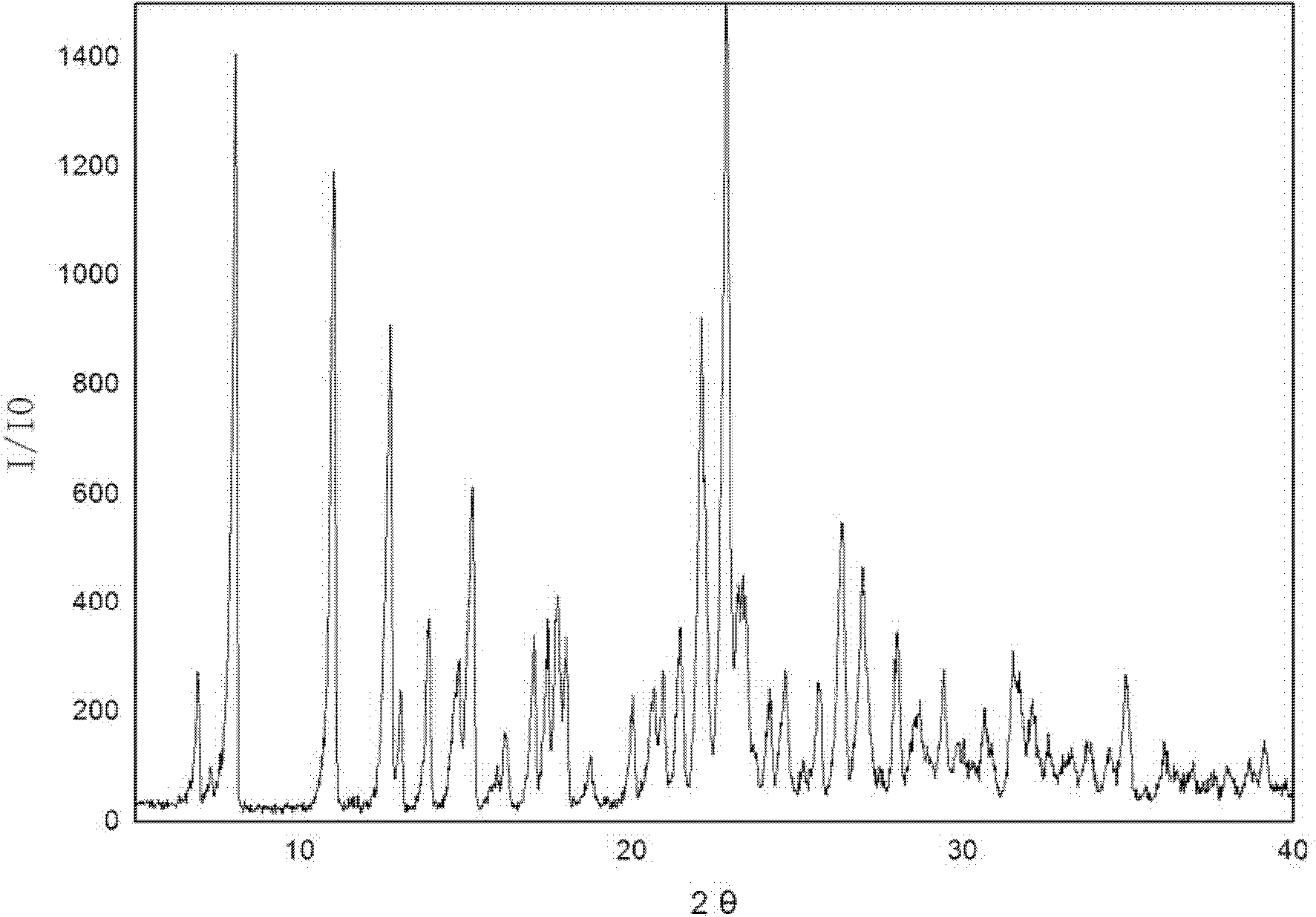
[0023] 3g of irbesartan was added to the crystallizer, and 26mL of acetone was added to dissolve the irbesartan. Add 1.97 g of 40% hydrobromic acid, stir to make the reaction complete, and the solution is clear. Start to add cyclohexane dropwise until the crystals come out, stop the dropwise addition, grow the crystals for 15-20 minutes, then continue to add cyclohexane, the amount of cyclohexane added is 250mL in total. Suction filtration, washing with water. The product was dried at 90°C for 5 hours to obtain irbesartan hydrobromide, wherein the mass percentage of water was 3.5%, and the powder diffraction results of the product were as follows: figure 2 shown. There are two endothermic peaks in DSC differential scanning calorimetry, and the corresponding peak top temperatures are 138.0°C and 198.3°C, respectively.
Embodiment 4
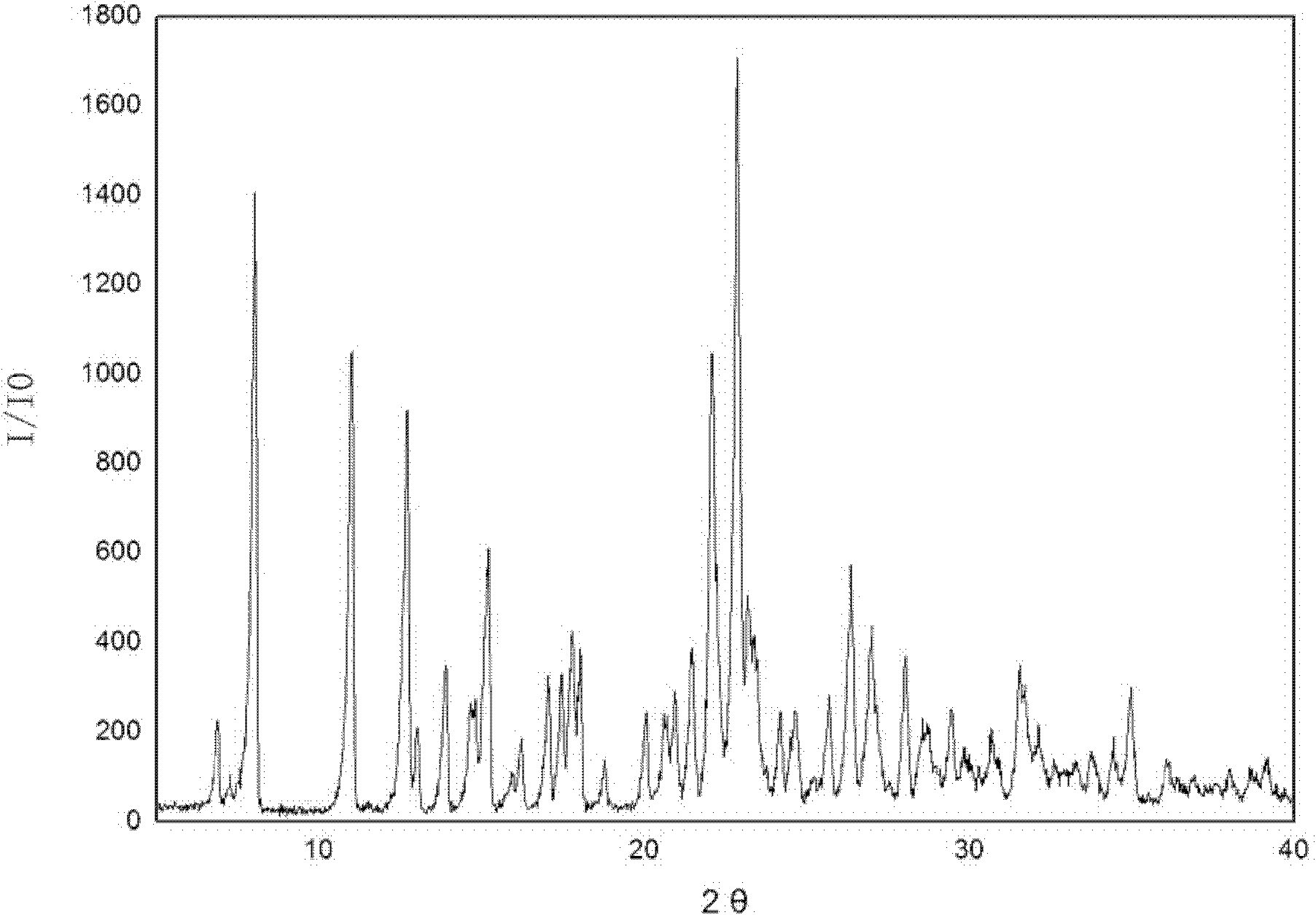
[0025] 5g of irbesartan was added to the crystallizer, 20mL of dioxane was added, and irbesartan was dissolved in the solvent. Add 2.36 g of 40% hydrobromic acid and stir to complete the reaction. Start to add ether 50mL dropwise. Suction filtration, washing with water. The product was dried at 50°C for 5 hours to obtain irbesartan hydrobromide, wherein the mass percentage of water was 5%, and the position of the characteristic peak obtained by X-ray powder diffraction of the product was expressed in 2θ as: at 8.15, 11.07, 12.63, 14.61, 15.13, 22.16, 22.88, 23.26, 26.48, 31.55, and also contain characteristic peaks at diffraction angles 2θ°=7.35, 13.99, 17.58, 17.97, 21.5, 28.1, 29.53. There are two endothermic peaks in DSC differential scanning calorimetry, and the corresponding peak temperatures are 140.0°C and 198.3°C, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com