Method for producing m-chlorobenzoyl chloride
A technology for the production of chlorobenzoyl chloride, which is applied in the field of producing m-chlorobenzoyl chloride, can solve the problems of high cost of raw materials, and achieve the effects of reduced production cost, high yield, and safe and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
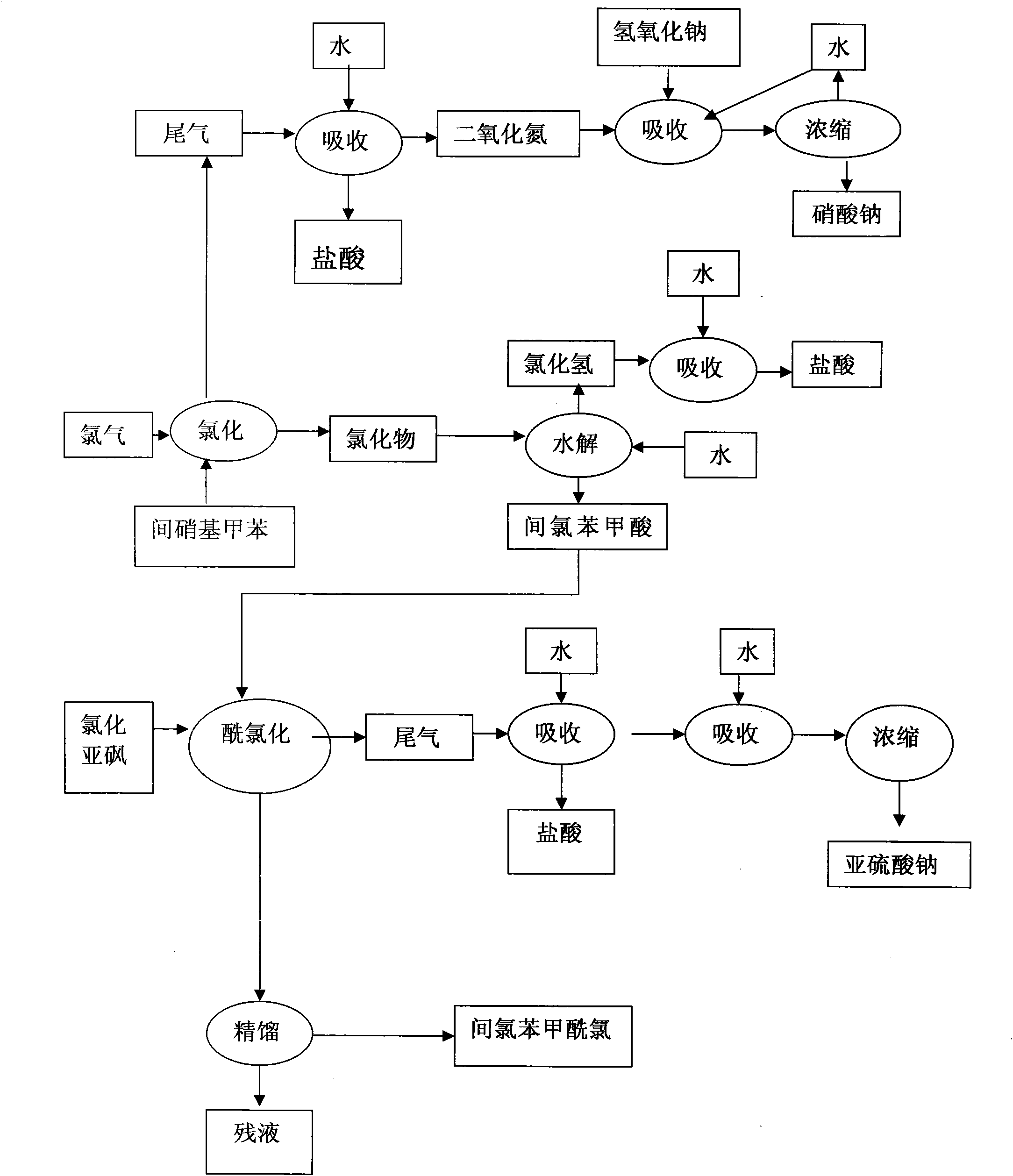
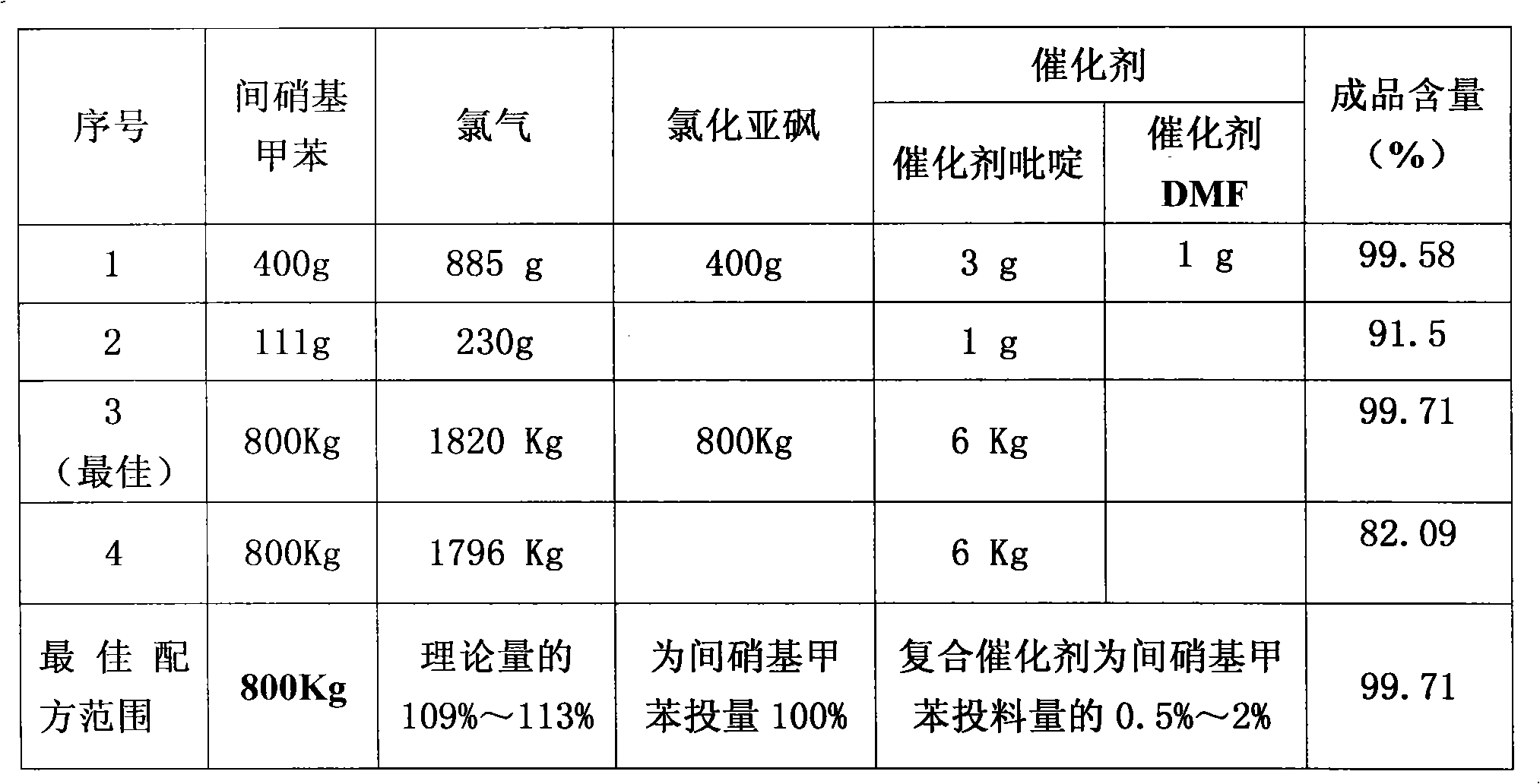
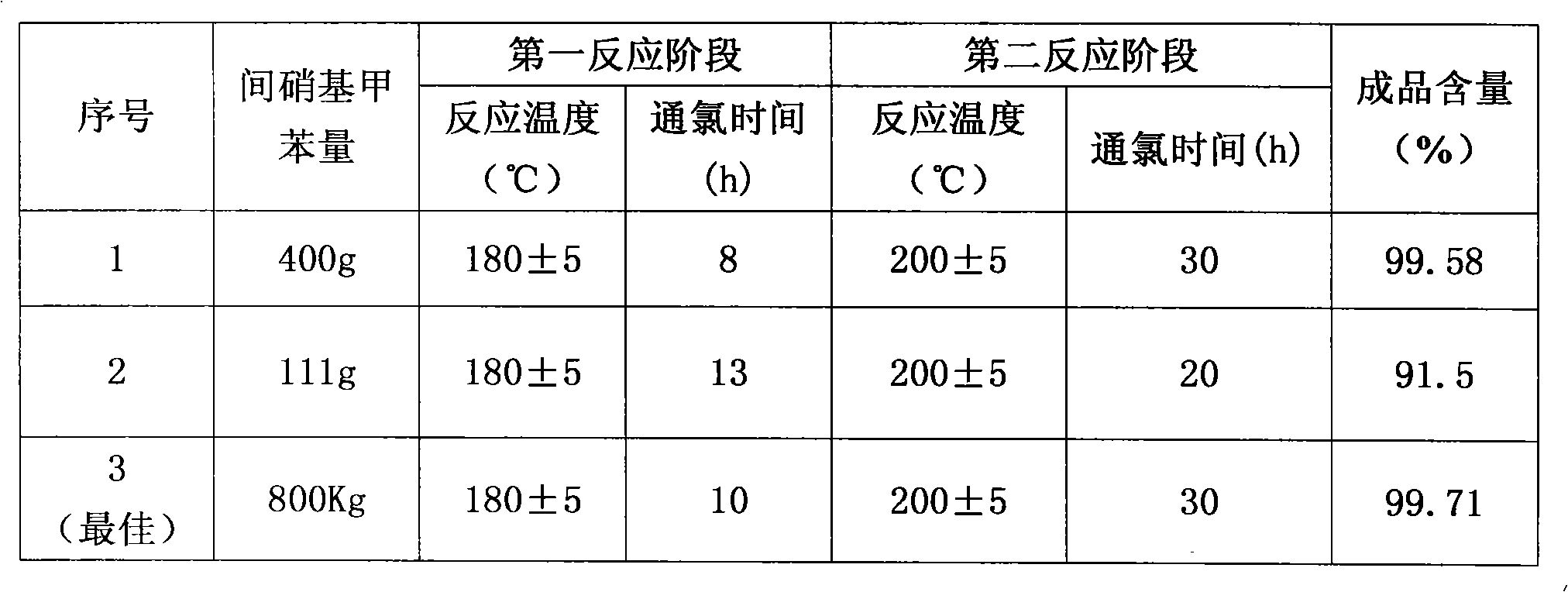
[0029] Add 400 grams of m-nitrotoluene, 3 grams of catalyst pyridine, and 1 gram of catalyst N,N-dimethylformamide (DMF) into a 500 ml three-necked flask, and heat the material to 180 degrees under ultraviolet light irradiation. Keep flowing chlorine continuously and slowly in the bottle at 180 degrees for 8 hours, then continue to pass chlorine at 200 degrees for 30 hours, with a total chlorine consumption of 885 grams. Obtain 496.3 grams of chloride, and through gas chromatography analysis, the content of m-chlorobenzoyl chloride is 92.3%. Chloride was hydrolyzed and crystallized in xylene to obtain 386 grams of m-chlorobenzoic acid. The m-chlorobenzoic acid was reacted with thionyl chloride and rectified to obtain 408 grams of m-chlorobenzoyl chloride with a content of 99.58%.
Embodiment 2
[0031] Add 111 grams of m-nitrotoluene and 1 gram of catalyst pyridine into a 250 milliliter three-necked flask, and heat the material to 180 degrees under ultraviolet light irradiation. Keep flowing chlorine continuously and slowly for 13 hours in the bottle at 180 degrees, then continue to pass chlorine for 20 hours at 200 degrees, with a total chlorine consumption of 230 grams. Obtain 136.5 grams of chloride, and through gas chromatography analysis, the m-chlorobenzoyl chloride content is 91.5%.
Embodiment 3
[0033] Add 800 kg of m-nitrotoluene and 6 kg of catalyst pyridine into a 1000-liter glass-lined reaction pot, and heat the material to 150 degrees under ultraviolet light irradiation. Continue to pass chlorine continuously at 150 degrees for 1 hour, then continue to pass chlorine at 180 degrees for 10 hours, and continue to pass chlorine at 200 degrees for 30 hours, with a total chlorine consumption of 1820 kg. Obtain 1016 kilograms of chlorides, through gas chromatography analysis, m-chlorobenzoyl chloride content is 86.7%. The chloride was hydrolyzed, acid chlorinated and rectified to obtain 848.5 kg of m-chlorobenzoyl chloride with a content of 99.71%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com