Method for preparing multielement cathode materials for lithium ion batteries
A technology for lithium ion batteries and positive electrode materials, which is applied in battery electrodes, electrical components, circuits, etc., can solve the problems that it is difficult to meet the needs of large lithium ion power batteries, poor conductivity of lithium iron phosphate, and difficulty in practical application. High current charge and discharge capacity, good dispersion, and the effect of being beneficial to the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
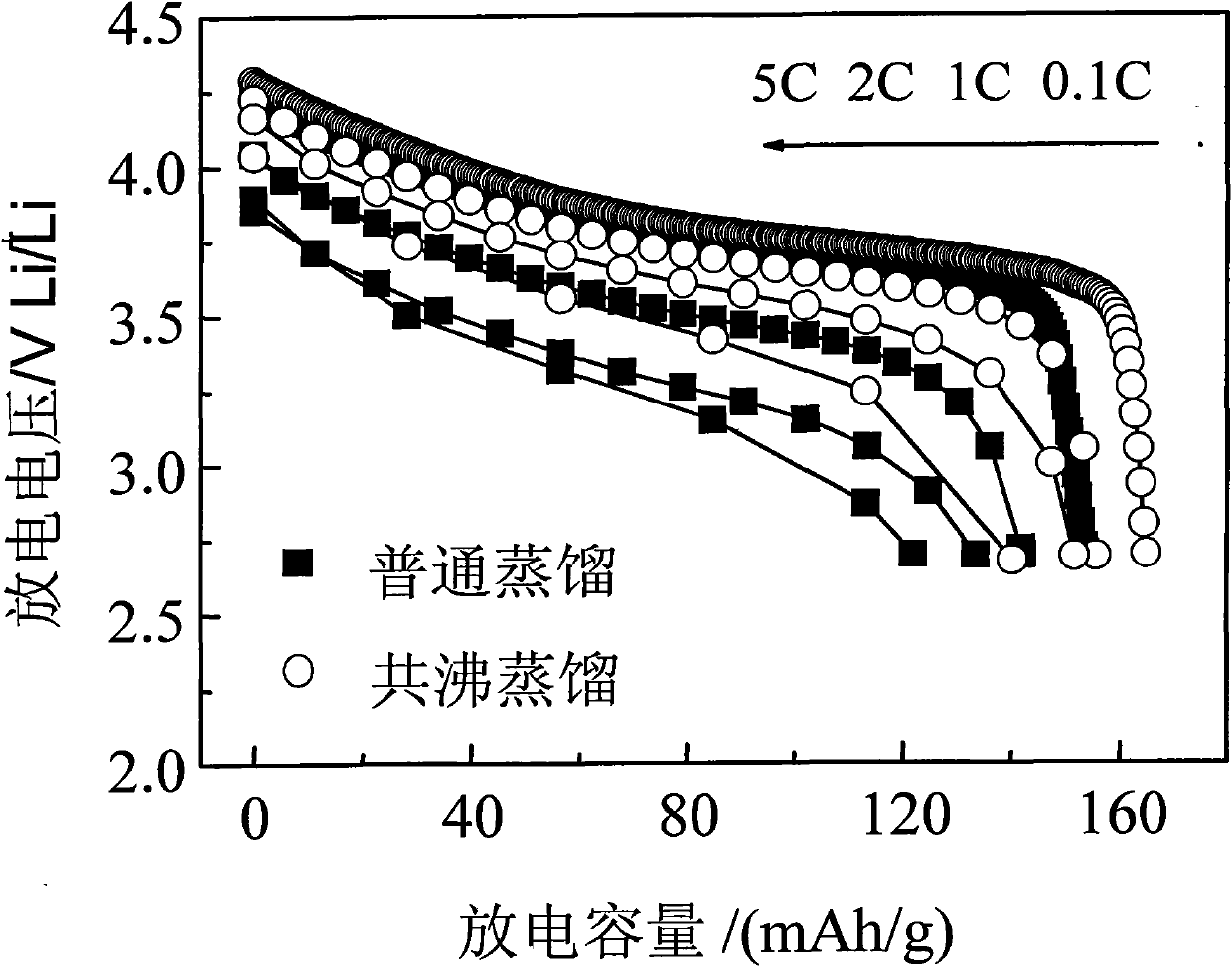
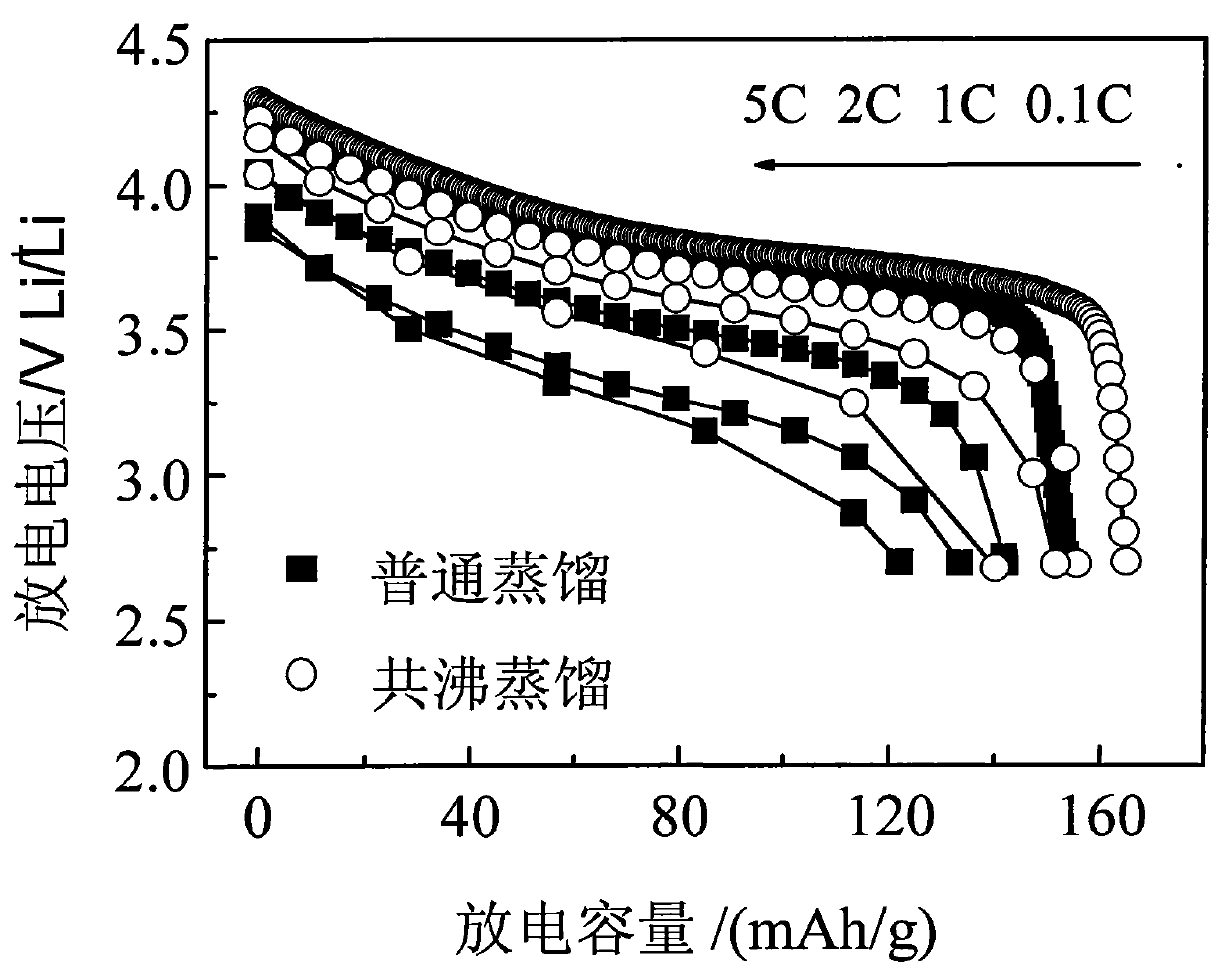
[0029] The multicomponent composite oxide lithium salt prepared in this embodiment is Li 1.1 Ni 1 / 3 co 1 / 3 mn 1 / 3 o 2 . First weigh NiSO according to the metal atomic ratio 4 、CoSO 4 , MnSO 4 , dissolved in deionized water to prepare a mixed solution with a total concentration of 1mol / L, then slowly add the above solution into the prepared 1mol / L NaOH solution, stir while adding, control the temperature at 80°C, and use NaOH solution Or dilute sulfuric acid to adjust pH=11, after reacting for 8 hours, filter the reaction mixture, wash the precipitate with deionized water and ethanol several times, then mix the precipitate obtained after cleaning with the corresponding proportion of LiOH·H 2 O is mixed evenly, then water and n-butanol (preferably in excess) are added to form an azeotropic system, stirred, azeotropically distilled and dried to obtain a precursor, the precursor is calcined at 400°C in oxygen or air for 5 hours, ground and sieved after cooling, and then Ca...
Embodiment 2
[0031] The multi-element composite oxide lithium salt prepared in this embodiment is LiNi 0.8 co 0.2 o 2 . First, weigh Ni(NO 3 ) 2 , Co(NO 3 ) 2 Dissolve in deionized water to prepare a mixed solution with a total concentration of 3mol / L, then slowly add the above solution into the prepared 2mol / L NH 4 OH solution, stirring while adding, the temperature is controlled at 55 ° C, with NH 4 OH solution or dilute nitric acid to adjust the pH to 12. After reacting for 10 hours, filter the reaction mixture, wash the precipitate with deionized water and ethanol several times, and then mix the cleaned precipitate with a corresponding proportion of LiOH·H 2 O mix evenly, add water, ethanol and benzene (ethanol and benzene are preferably in excess) to form an azeotropic system, stir, azeotropically distill and dry to obtain a precursor, calcinate the precursor at 450°C in oxygen or air for 6 hours, cool and grind After sieving, and then calcining at 900°C for 20 hours in an oxy...
Embodiment 3
[0033] The multicomponent composite oxide lithium salt prepared in this embodiment is Li 1.2 Ni 1 / 3 mn 2 / 3 o 2 . First, weigh Ni(NO 3 ) 2 , Mn(NO 3 ) 2 , dissolved in deionized water to prepare a mixed solution with a total concentration of 0.5mol / L, and then slowly add the above solution to the prepared 0.2mol / LNa 2 CO 3 solution, stirring while adding, the temperature was controlled at 75°C, and NH 4 OH solution or dilute nitric acid to adjust pH=6, after reacting for 6 hours, filter the reaction mixture, wash the precipitate with deionized water and ethanol several times, and then mix the washed precipitate with the corresponding proportion of Li 2 CO 3 Mix evenly, add water and isoamyl alcohol (preferably in excess) to form an azeotropic system, stir, azeotropically distill and dry to obtain a precursor, calcinate the precursor at 450°C in oxygen or air for 5 hours, grind and sieve after cooling, and then Calcined at 650°C for 5 hours in an oxygen or air atmosph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com