Preparation method of donepezil
A technology of donepezil and production method, which is applied in the field of donepezil production technology, can solve the problems of product purity decrease, high price, poor reduction stability, etc., and achieve the effects of product purity improvement, small equipment investment and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
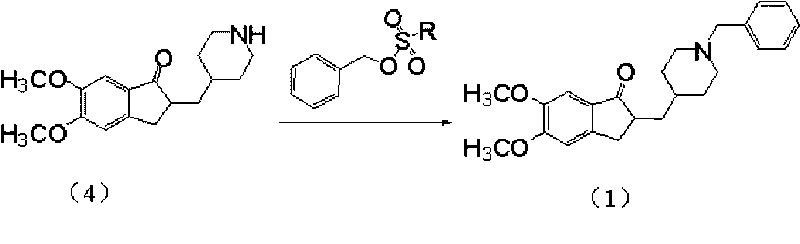
[0027] Hydrate (4) 28.9g (0.1mol), sodium bicarbonate 12.6g (0.15mol), tetrabutylammonium bromide 1.6g (5.0mmol), benzyl methanesulfonate 19.5g (0.105mol) and 300ml formazan mixed with isobutyl ketone, heated to 50°C, reacted for 4 hours, cooled to room temperature, successively washed with 1% (wt) sodium hydroxide aqueous solution and water, dried over anhydrous sodium sulfate, filtered off the desiccant and evaporated to dryness 35 g of white solid was obtained, yield 92.3%, melting point: 91-93° C., HPLC>97.5%.
[0028] MS(TOF): 380(M + +H + );402(M + +K + )
[0029] 1 HNMR (400Hz, CDCl 3 )δ: 7.23-7.32 (5H, m), 7.16 (1H, s), 6.85 (1H, s), 3.90-3.95 (6H, s), 3.50 (2H, s), 3.19-3.26 (1H, m) , 2.87-2.92(2H, m), 2.66-2.72(2H, m), 1.88-2.01(3H, m), 1.64-1.75(2H, m), 1.46-1.51(1H, m), 1.37-1.42( 3H, m).
Embodiment 2
[0031] The hydride (4) 28.9g (0.1mol), sodium bicarbonate 12.6g (0.15mol), tetramethylammonium bromide 0.77g (5.0mmol), benzyl methanesulfonate 19.5g (0.105mol) and 300ml formazan mixed with isobutyl ketone, reacted at 0°C for 5 hours, then washed with 1% (wt) aqueous sodium hydroxide solution, washed with water, dried over anhydrous sodium sulfate, filtered off the desiccant and evaporated to dryness to obtain 34.4 g of a white solid. The yield was 90.8%, and HPLC>97.5%.
Embodiment 3
[0033] Hydride (4) 28.9g (0.1mol), potassium bicarbonate 15.0g (0.15mol), tetramethylammonium chloride 0.77g (5.0mmol), benzyl methanesulfonate 19.5g (0.105mol) and 300ml formazan Base isobutyl ketone mixed, reflux reaction for 2 hours, then after washing with 1% (wt) aqueous sodium hydroxide solution, water, anhydrous sodium sulfate, filter off the desiccant and evaporate the solvent to dryness to obtain white solid 34.5g, yield 91.0 %, HPLC>97.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com