Method for preparing cefmenoxime hydrochloride freeze-dried powder injection
A technology of cefmenoxime hydrochloride and freeze-dried powder injection, which is applied in the field of preparation of cefmenoxime hydrochloride freeze-dried powder injection, can solve the problems of complex preparation, unstable quality, high cost, etc., and achieve the effect of improving stability and dissolution speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
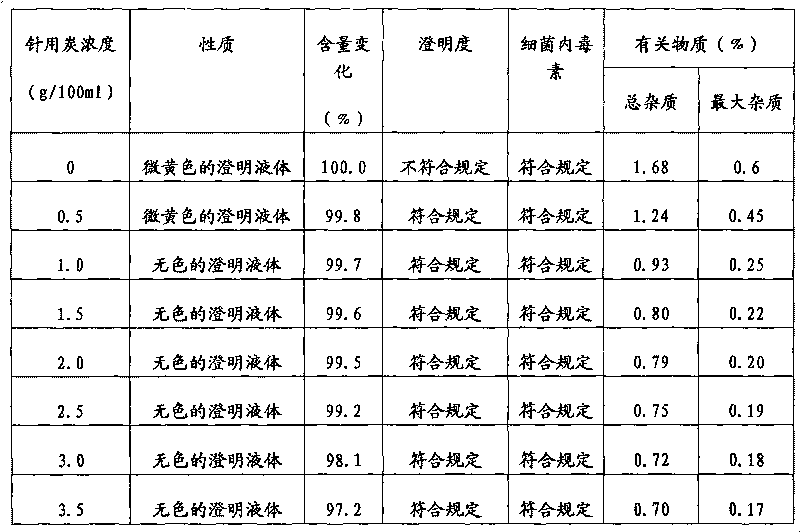
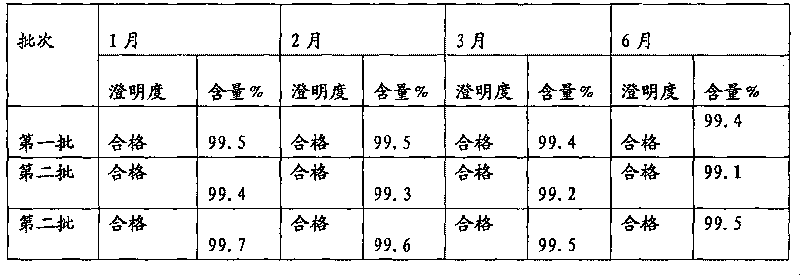
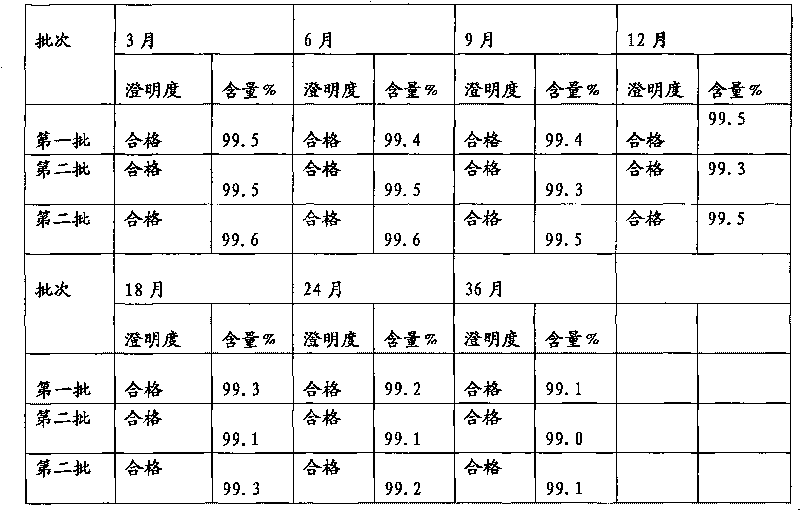
[0026] Dissolve 40 g of cefmenoxime hydrochloride finished product and 4 g of arginine in 100 ml of fresh water for injection, stir to dissolve, add 1 g of activated carbon, stir for decolorization for 30 minutes, and filter aseptically. Put the filtrate into a lyophilizer after subpackaging, quickly cool down to -40°C, keep at -40°C for 3 hours, vacuumize, gradually increase the temperature to -20°C within 8 hours, and maintain -20°C for 8 hours in vacuum , continue to heat up to 25° C. within 2 hours, maintain 25° C. and dry in vacuum until the degree of vacuum does not change, to obtain cefmenoxime hydrochloride freeze-dried powder injection.
Embodiment 2
[0028] Dissolve 40 g of cefmenoxime hydrochloride finished product and 10 g of arginine in 100 ml of fresh water for injection, stir to dissolve, add 2.5 g of activated carbon, stir for 30 minutes to decolorize, and filter aseptically. Put the filtrate into a lyophilizer after subpackaging, quickly cool down to -30°C, keep at -30°C for 5 hours, vacuumize, gradually increase the temperature to -15°C within 6 hours, and maintain -20°C for 8 hours in vacuum , continue to heat up to 30 DEG C within 4 hours, maintain 30 DEG C and vacuum-dry until the vacuum degree does not change, to obtain cefmenoxime hydrochloride freeze-dried powder injection.
Embodiment 3
[0030] Dissolve 40 g of cefmenoxime hydrochloride finished product and 20 g of arginine in 100 ml of freshly prepared water for injection, stir to dissolve, add 2 g of activated carbon, stir for decolorization for 30 minutes, and filter aseptically. Put the filtrate into a lyophilizer after subpackaging, quickly cool down to -35°C, maintain -40°C for 4 hours, vacuumize, gradually increase the temperature to -20°C within 8 hours, and maintain -15°C for 10 hours in vacuum , continue to heat up to 25°C within 3 hours, maintain 25°C and vacuum-dry until the degree of vacuum does not change, to obtain cefmenoxime hydrochloride freeze-dried powder injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com