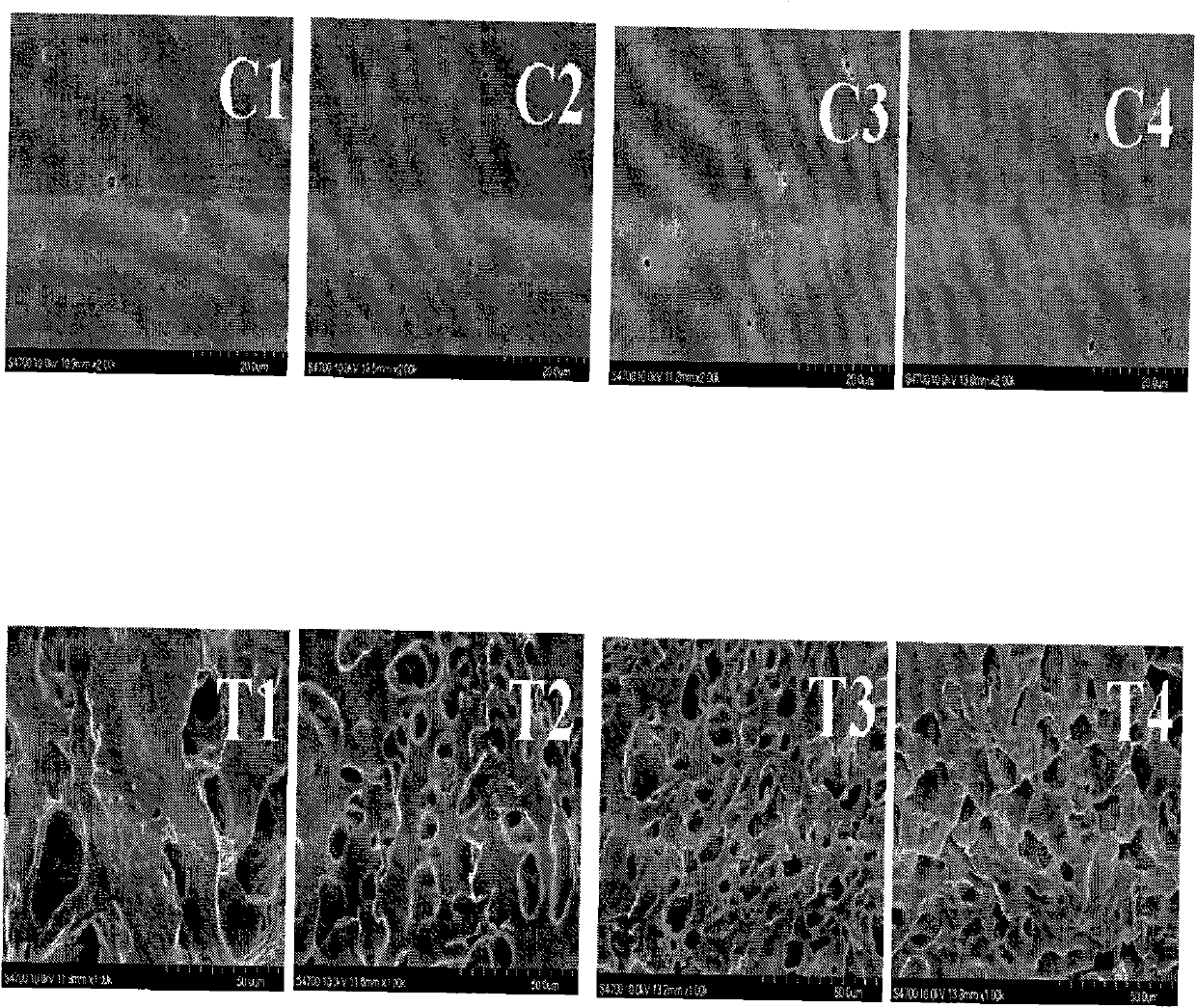
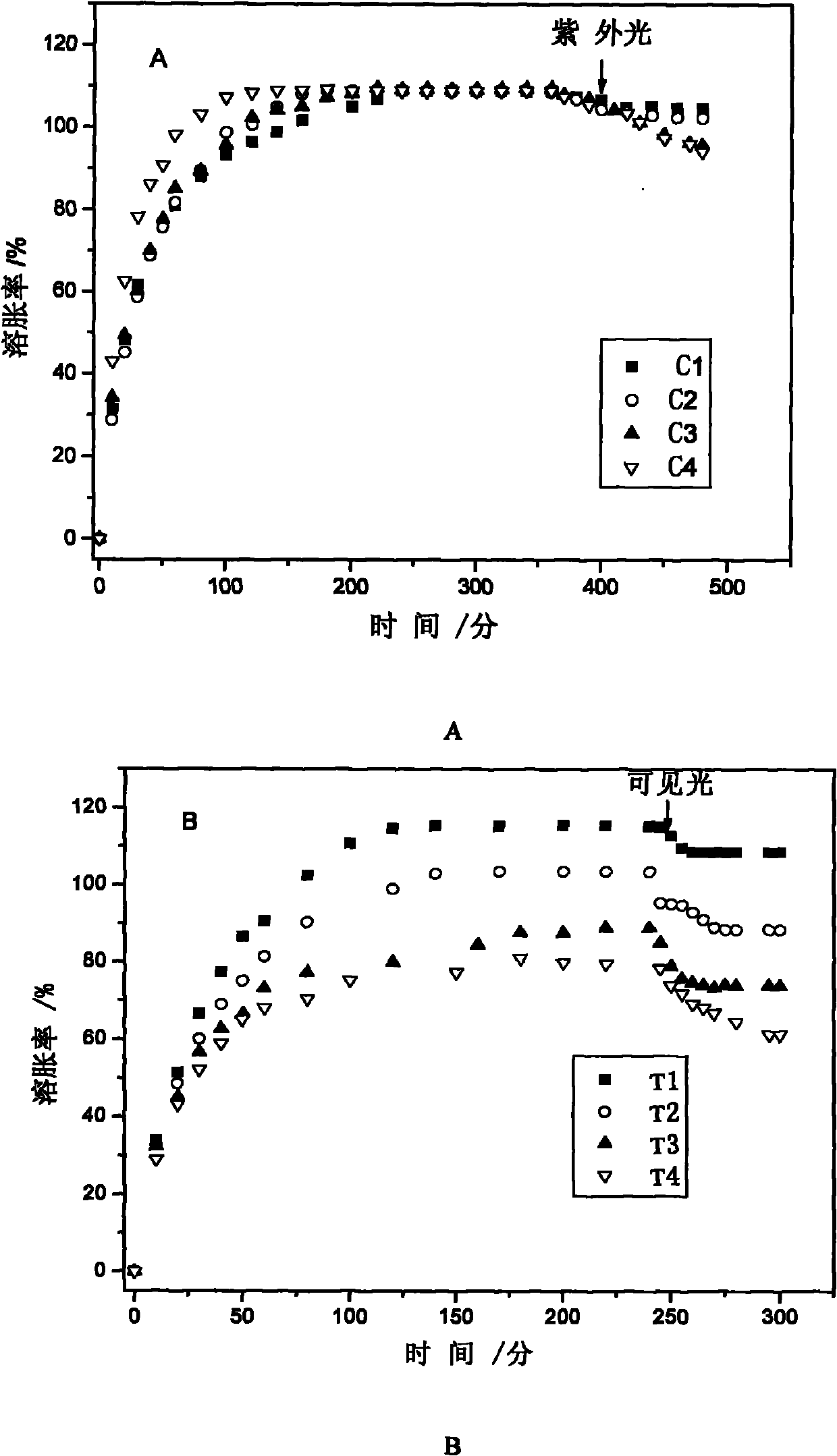
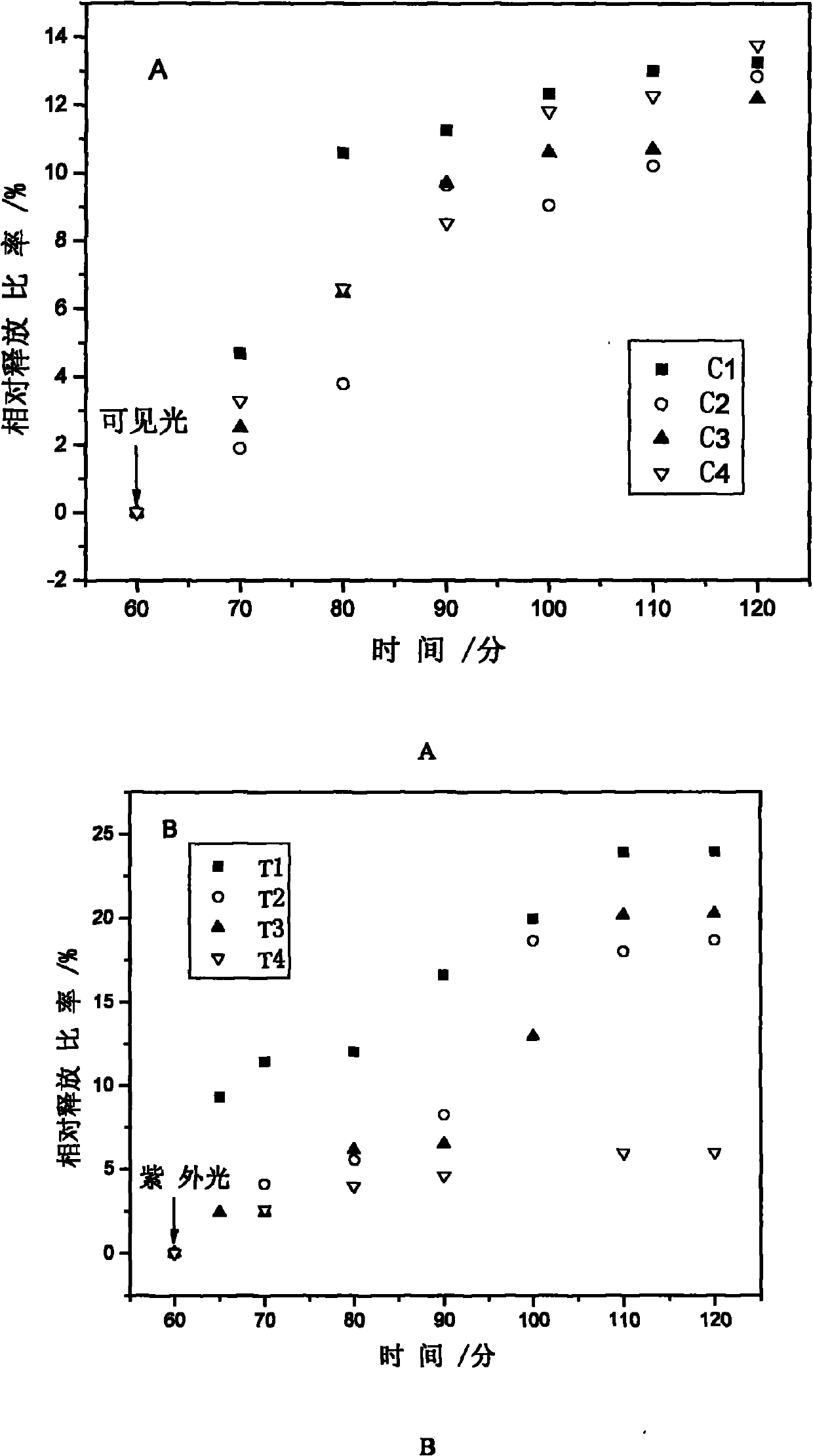
Method for preparing photoresponse hydrogel containing azo monomer by utilizing light sources with different wavelengths
A light-responsive, hydrogel technology, applied in the field of light-responsive hydrogels, can solve problems such as the adverse effects of restricted thermal diffusion and restricted hydrogen ion diffusion, and achieve mild conditions and simple steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of azo monomer as 4-sulfonic acid-4'acryloyloxyazobenzene hydrogel
[0021] (1) Sonicate four mixed solutions of HEA (1.5g), PEGDA-600 (0.15g), N, N methylenebisacrylamide (0.15g) and TPO (0.075g) with the same content for 10 minutes, and add 4-Sulphono-4'acryloyloxyazobenzene (0.015g, 0.03g, 0.045g, 0.06g) and water (3g). Irradiate (2 minutes, 5 minutes, 10 minutes, 30 minutes) respectively with ultraviolet lamp (50mw). The prepared colloid was washed with water for 5 times, and placed in a vacuum oven at 40° C. for 24 hours.
[0022] (2) Four parts of the same content of HEA (1.5g), PEGDA-600 (0.15g), N, N methylenebisacrylamide (0.15g), camphorquinone (CQ) and p-N, N-di The mixed solution of ethyl methylaminobenzoate (EDMAB) (0.075g) was ultrasonicated for 10 minutes, and 4-sulfonic acid-4'acryloyloxyazobenzene (0.015g, 0.03g, 0.045g, 0.06g ) and water (3g). Irradiate (5 minutes, 10 minutes, 30 minutes, 60 minutes) respectively with a visib...
Embodiment 2
[0025] Example 2: Preparation of azo monomer as 4-sulfonic acid-4'methacryloyloxyazobenzene hydrogel
[0026] (1) Sonicate four mixed solutions of HEMA (1.5g), PEGDA-400 (0.075g), N, N methylenebisacrylamide (0.075g) and TPO (0.0075g) with the same content for 10 minutes, and add 4-Sulphono-4'acryloyloxyazobenzene (0.015g, 0.03g, 0.045g, 0.06g) and water (4.5g). Use ultraviolet light (80mw) to irradiate (2 minutes, 5 minutes, 10 minutes, 30 minutes) respectively. The prepared colloid was washed with water for 5 times, and placed in a vacuum oven at 40° C. for 24 hours.
[0027] (2) Four parts of the same content of HEA (1.5g), PEGDA-400 (0.075g), N, N methylenebisacrylamide (0.075g), camphorquinone (CQ) and p-N, N-di The mixed solution of ethyl methylaminobenzoate (EDMAB) (0.0075g) was sonicated for 10 minutes, and 4-sulfonic acid-4'methacryloyloxyazobenzene (0.015g, 0.03g, 0.045g, 0.06g) and water (4.5g). Irradiate (5 minutes, 10 minutes, 30 minutes, 60 minutes) respectiv...
Embodiment 3
[0030] Example 3: Preparation of azo monomer as 4-carboxy-4'-acryloyloxyazobenzene hydrogel
[0031] (1) Sonicate five mixed solutions of the same content of HEA (1.5g), PEGDA-200 (0.225g), N, N methylenebisacrylamide (0.225g), TPO (0.03g) for 10 minutes, add 2-cyano-4-sulfo-2'-carboxy-4'-acryloxyazobenzene (0.015g, 0.03g, 0.045g, 0.06g, 0.075g) and water (6g). Irradiate (2 minutes, 5 minutes, 10 minutes, 15 minutes, 30 minutes) with ultraviolet lamp (120mw). The prepared colloid was washed with water for 5 times, and placed in a vacuum oven at 40° C. for 24 hours.
[0032] (2) Five parts of the same content of HEA (1.5g), PEGDA-200 (0.225g), N, N methylenebisacrylamide (0.225g), camphorquinone (CQ) and p-N, N-di The mixed solution of ethyl methylaminobenzoate (EDMAB) (0.03g) was ultrasonicated for 10 minutes, and 4-carboxy-4'-acryloyloxyazobenzene (0.015g, 0.03g, 0.045g, 0.06g, 0.075g) and water (6g). Irradiate (5 minutes, 10 minutes, 15 minutes, 30 minutes, 60 minutes) w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com