Process for preparing caprolactam
A technology for preparing caprolactam, which is applied in the field of preparing caprolactam, can solve the problems of complex process, large equipment investment, and long process flow, and achieve the effects of simplified process flow, shortened process flow, and strong extraction capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
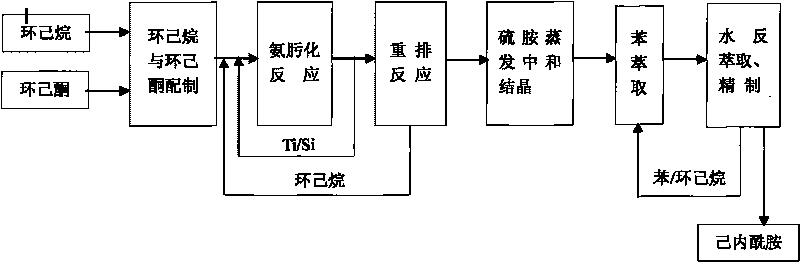
[0042] Embodiment 1: as figure 1 As shown, 99.88% (wt) cyclohexanone from the cyclohexanone preparation process and 99.9% cyclohexane from the same process are formulated into 20-30% cyclohexanone at a volume ratio of 1:3-1:4 The cyclohexane solution, then enters the ammoximation reactor at a flow rate of 40.4-50.5t / h.
[0043] Add 2.0~2.2t / h of NH to the ammonia oximation reactor at the same time 3 , 15.1~15.7t / hH 2 o 2 , in the presence of a Ti / Si molecular sieve catalyst with a concentration of 2-4% (wt), at a temperature of 80-90 ° C and a pressure of 0.4 MPa, the ammoximation reaction is carried out under normal pressure conditions, and then filtered out by hydrocyclone separation The water phase containing the catalyst is controlled to pass through the coalescer to remove a small amount of brine under a flow rate of 42.5t / h to 52.5t / h to obtain a cyclohexane solution of cyclohexanone oxime.
[0044] The cyclohexane solution of cyclohexanone oxime enters the Beckmann ...
Embodiment 2
[0049] Embodiment 2: the difference between this embodiment and embodiment 1 is that the toluene extractant replaces the benzene extractant.
Embodiment 3
[0050] Embodiment 3: the difference between this embodiment and embodiment 1 is that the trichlorethylene extractant replaces the benzene extractant.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com